Abstract
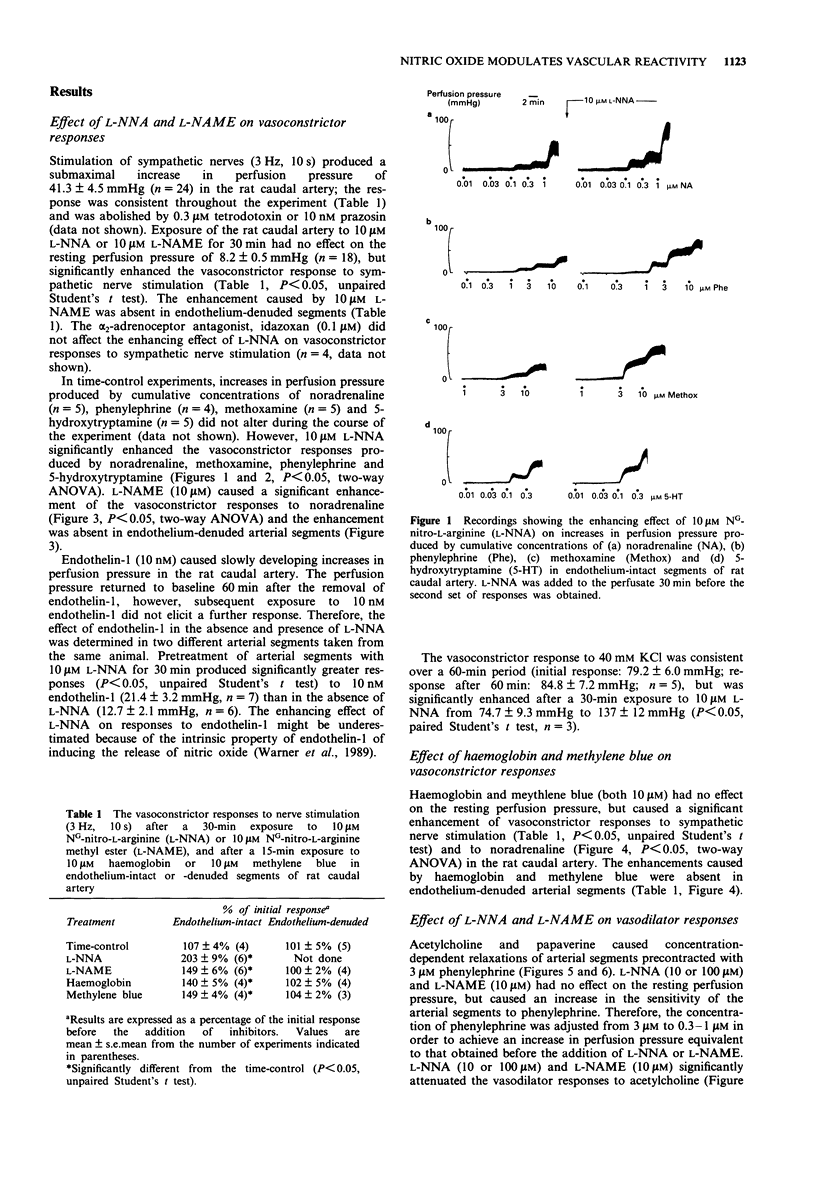
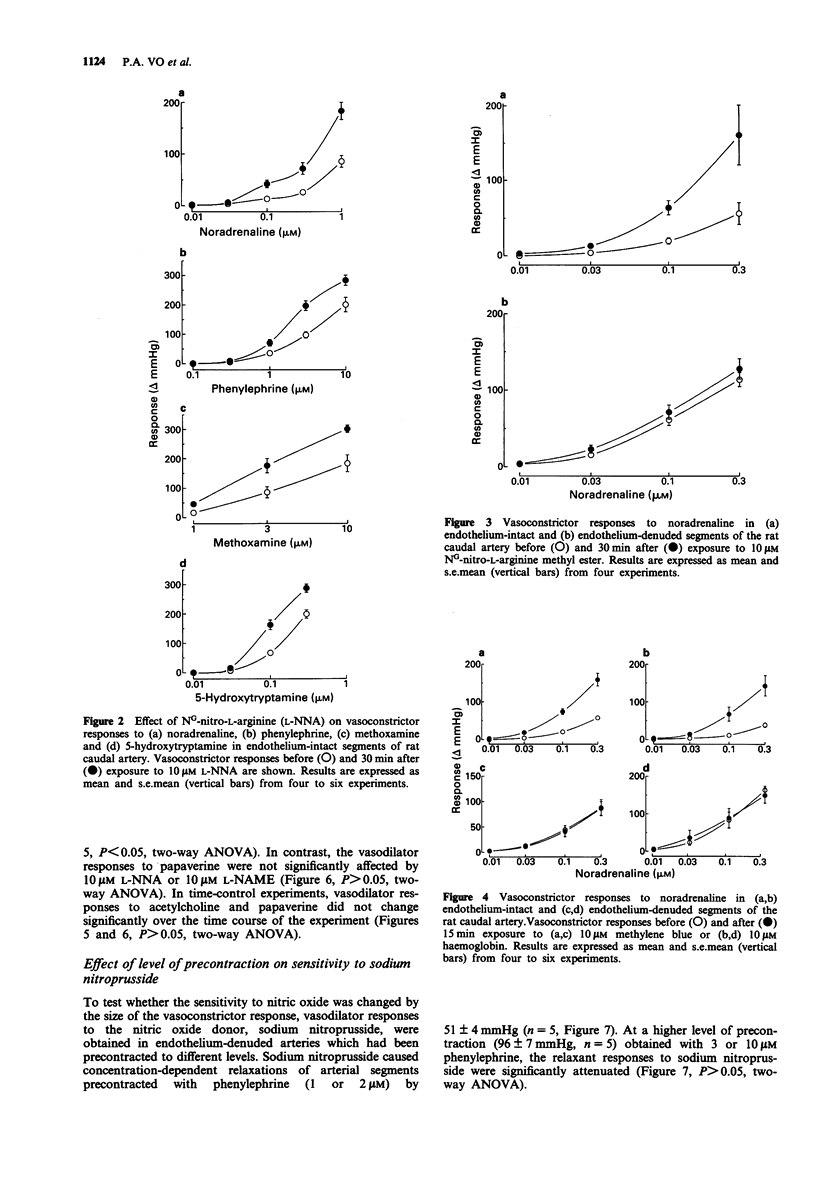
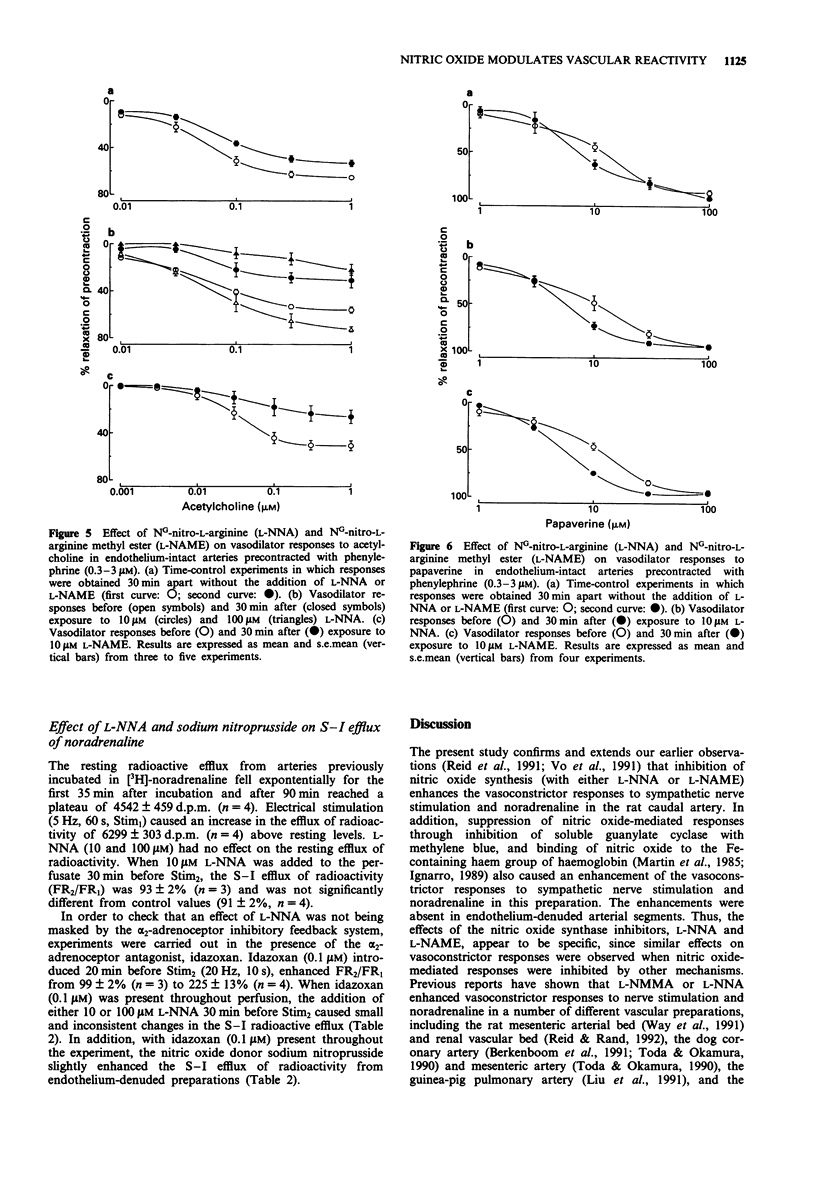
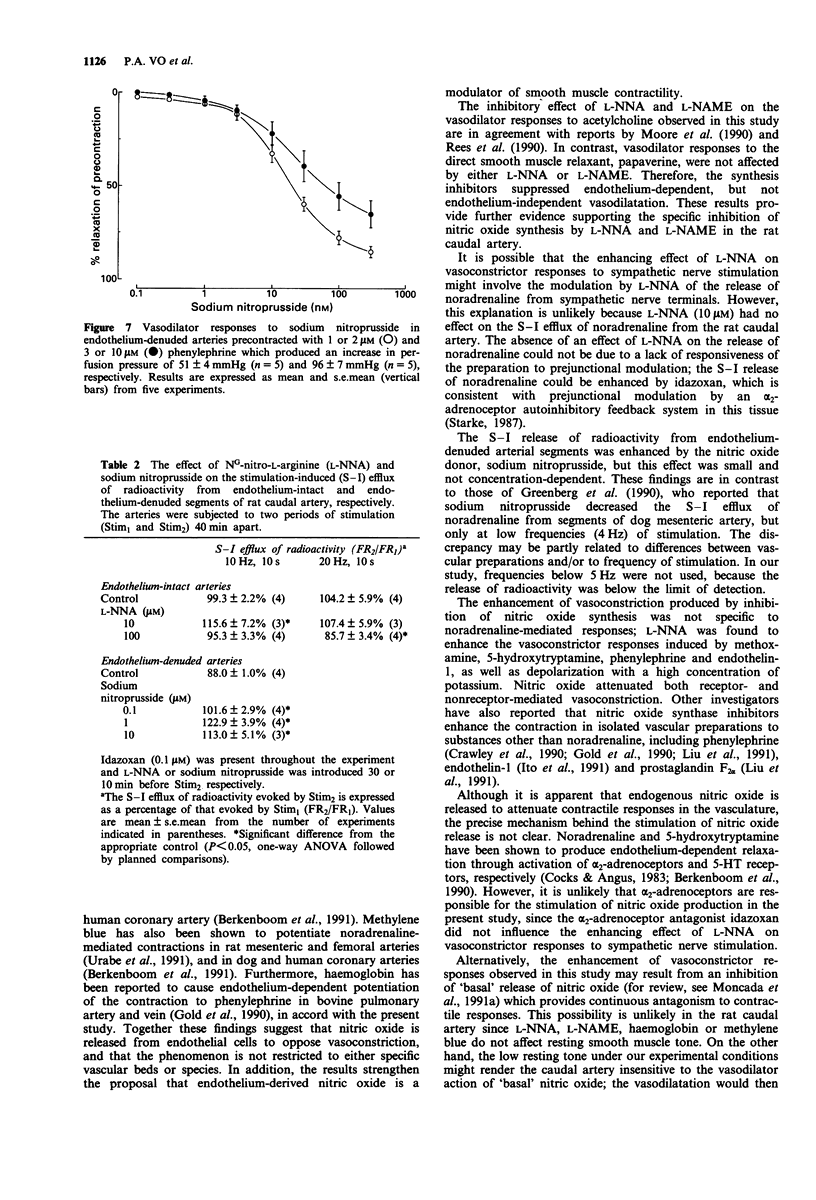
1. The effects of NG-nitro-L-arginine (L-NNA), NG-nitro-L-arginine methyl ester (L-NAME), haemoglobin and methylene blue have been examined on vascular reactivity in the rat isolated caudal artery. The effects of L-NNA and sodium nitroprusside were also investigated on the stimulation-induced (S-I) efflux of noradrenaline in the rat caudal artery. 2. L-NNA (10 microM) and L-NAME (10 microM) significantly attenuated the vasodilator responses to acetylcholine (1 nM-1 microM), but had no effect on vasodilator responses to papaverine (1-100 microM). 3. Vasoconstrictor responses to sympathetic nerve stimulation (3 Hz, 10 s), noradrenaline (0.01-1 microM), methoxamine (1-10 microM), 5-hydroxytryptamine (0.01-0.3 microM), phenylephrine (0.1-10 microM), endothelin-1 (10 nM) and KCl (40 mM) were significantly enhanced by 10 microM L-NNA. L-NAME (10 microM) caused a significant enhancement of vasoconstrictor responses to noradrenaline and sympathetic nerve stimulation in endothelium-intact, but not in endothelium-denuded tissues. 4. Haemoglobin and methylene blue (both 10 microM) enhanced the vasoconstrictor responses to sympathetic nerve stimulation and noradrenaline. The enhancements were absent in endothelium-denuded arterial segments. 5. In endothelium-denuded arterial segments precontracted with phenylephrine, the vasodilator responses to the nitric oxide donor, sodium nitroprusside (0.1-300 nM) were decreased by increasing the level of precontraction. 6. L-NNA (10 microM) had no effect on the S-I efflux of radioactivity from arteries in which transmitter stores had been labelled with [3H]-noradrenaline.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buga G. M., Gold M. E., Fukuto J. M., Ignarro L. J. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991 Feb;17(2):187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983 Oct 13;305(5935):627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- Crawley D. E., Liu S. F., Evans T. W., Barnes P. J. Inhibitory role of endothelium-derived relaxing factor in rat and human pulmonary arteries. Br J Pharmacol. 1990 Sep;101(1):166–170. doi: 10.1111/j.1476-5381.1990.tb12107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Gold M. E., Wood K. S., Byrns R. E., Fukuto J., Ignarro L. J. NG-methyl-L-arginine causes endothelium-dependent contraction and inhibition of cyclic GMP formation in artery and vein. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4430–4434. doi: 10.1073/pnas.87.12.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. S., Diecke F. P., Cantor E., Peevy K., Tanaka T. P. Inhibition of sympathetic neurotransmitter release by modulators of cyclic GMP in canine vascular smooth muscle. Eur J Pharmacol. 1990 Oct 23;187(3):409–423. doi: 10.1016/0014-2999(90)90368-g. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Ito S., Juncos L. A., Nushiro N., Johnson C. S., Carretero O. A. Endothelium-derived relaxing factor modulates endothelin action in afferent arterioles. Hypertension. 1991 Jun;17(6 Pt 2):1052–1056. doi: 10.1161/01.hyp.17.6.1052. [DOI] [PubMed] [Google Scholar]
- Lamontagne D., Pohl U., Busse R. Mechanical deformation of vessel wall and shear stress determine the basal release of endothelium-derived relaxing factor in the intact rabbit coronary vascular bed. Circ Res. 1992 Jan;70(1):123–130. doi: 10.1161/01.res.70.1.123. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Liu S. F., Crawley D. E., Evans T. W., Barnes P. J. Endogenous nitric oxide modulates adrenergic neural vasoconstriction in guinea-pig pulmonary artery. Br J Pharmacol. 1991 Oct;104(2):565–569. doi: 10.1111/j.1476-5381.1991.tb12469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Medgett I. C., Langer S. Z. Heterogeneity of smooth muscle alpha adrenoceptors in rat tail artery in vitro. J Pharmacol Exp Ther. 1984 Jun;229(3):823–830. [PubMed] [Google Scholar]
- Medgett I. C., Langer S. Z. Influence of neuronal uptake on the contribution of smooth muscle alpha 2-adrenoceptors to vasoconstrictor responses to noradrenaline in SHR and WKY isolated tail arteries. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):43–49. doi: 10.1007/BF00633195. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S., Rees D. D., Schulz R., Palmer R. M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M., Ochiai M., Taguchi J., Hara K., Akatsuka N., Kurokawa K. Stretch may enhance the release of endothelium-derived relaxing factor in rabbit aorta. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1038–1042. doi: 10.1016/s0006-291x(05)80890-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rajanayagam M. A., Musgrave I. F., Rand M. J., Majewski H. Facilitation of noradrenaline release by isoprenaline is not mediated by angiotensin II in mouse atria and rat tail artery. Arch Int Pharmacodyn Ther. 1989 May-Jun;299:185–199. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. J., Rand M. J. Renal vasoconstriction is modulated by nitric oxide. Clin Exp Pharmacol Physiol. 1992 May;19(5):376–379. doi: 10.1111/j.1440-1681.1992.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Reid J. J., Vo P. A., Lieu A. T., Wong-Dusting H. K., Rand M. J. Modulation of norepinephrine-induced vasoconstriction by endothelin-1 and nitric oxide in rat tail artery. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S272–S275. doi: 10.1097/00005344-199100177-00077. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Romero J. C., Vanhoutte P. M. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986 Jun;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokas E. G., Folco G. C. Intima-related vasodilatation of the perfused rat caudal artery. Eur J Pharmacol. 1984 Apr 20;100(2):211–217. doi: 10.1016/0014-2999(84)90225-5. [DOI] [PubMed] [Google Scholar]
- Starke K. Presynaptic alpha-autoreceptors. Rev Physiol Biochem Pharmacol. 1987;107:73–146. [PubMed] [Google Scholar]
- Takayanagi R., Ohnaka K., Takasaki C., Ohashi M., Nawata H. Multiple subtypes of endothelin receptors in human and porcine tissues: characterization by ligand binding, affinity labeling, and regional distribution. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S127–S130. doi: 10.1097/00005344-199100177-00034. [DOI] [PubMed] [Google Scholar]
- Thomas G., Cole E. A., Ramwell P. W. NG-monomethyl L-arginine is a non-specific inhibitor of vascular relaxation. Eur J Pharmacol. 1989 Oct 24;170(1-2):123–124. doi: 10.1016/0014-2999(89)90148-9. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Modification by L-NG-monomethyl arginine (L-NMMA) of the response to nerve stimulation in isolated dog mesenteric and cerebral arteries. Jpn J Pharmacol. 1990 Jan;52(1):170–173. doi: 10.1254/jjp.52.170. [DOI] [PubMed] [Google Scholar]
- Topouzis S., Schott C., Stoclet J. C. Participation of endothelium-derived relaxing factor and role of cyclic GMP in inhibitory effects of endothelium on contractile responses elicited by alpha-adrenoceptor agonists in rat aorta. J Cardiovasc Pharmacol. 1991 Nov;18(5):670–678. doi: 10.1097/00005344-199111000-00004. [DOI] [PubMed] [Google Scholar]
- Urabe M., Kawasaki H., Takasaki K. Effect of endothelium removal on the vasoconstrictor response to neuronally released 5-hydroxytryptamine and noradrenaline in the rat isolated mesenteric and femoral arteries. Br J Pharmacol. 1991 Jan;102(1):85–90. doi: 10.1111/j.1476-5381.1991.tb12136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Vo P. A., Reid J. J., Rand M. J. Endothelial nitric oxide attenuates vasoconstrictor responses to nerve stimulation and noradrenaline in the rat tail artery. Eur J Pharmacol. 1991 Jun 18;199(1):123–125. doi: 10.1016/0014-2999(91)90647-9. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Warner T. D., de Nucci G., Vane J. R. Rat endothelin is a vasodilator in the isolated perfused mesentery of the rat. Eur J Pharmacol. 1989 Jan 17;159(3):325–326. doi: 10.1016/0014-2999(89)90167-2. [DOI] [PubMed] [Google Scholar]
- Xiao X. H., Rand M. J. Alpha 2-adrenoceptor agonists enhance responses to certain other vasoconstrictor agonists in the rat tail artery. Br J Pharmacol. 1989 Mar;96(3):539–546. doi: 10.1111/j.1476-5381.1989.tb11851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Endothelin, a novel endothelium-derived peptide. Pharmacological activities, regulation and possible roles in cardiovascular control. Biochem Pharmacol. 1989 Jun 15;38(12):1877–1883. doi: 10.1016/0006-2952(89)90484-x. [DOI] [PubMed] [Google Scholar]


