Abstract
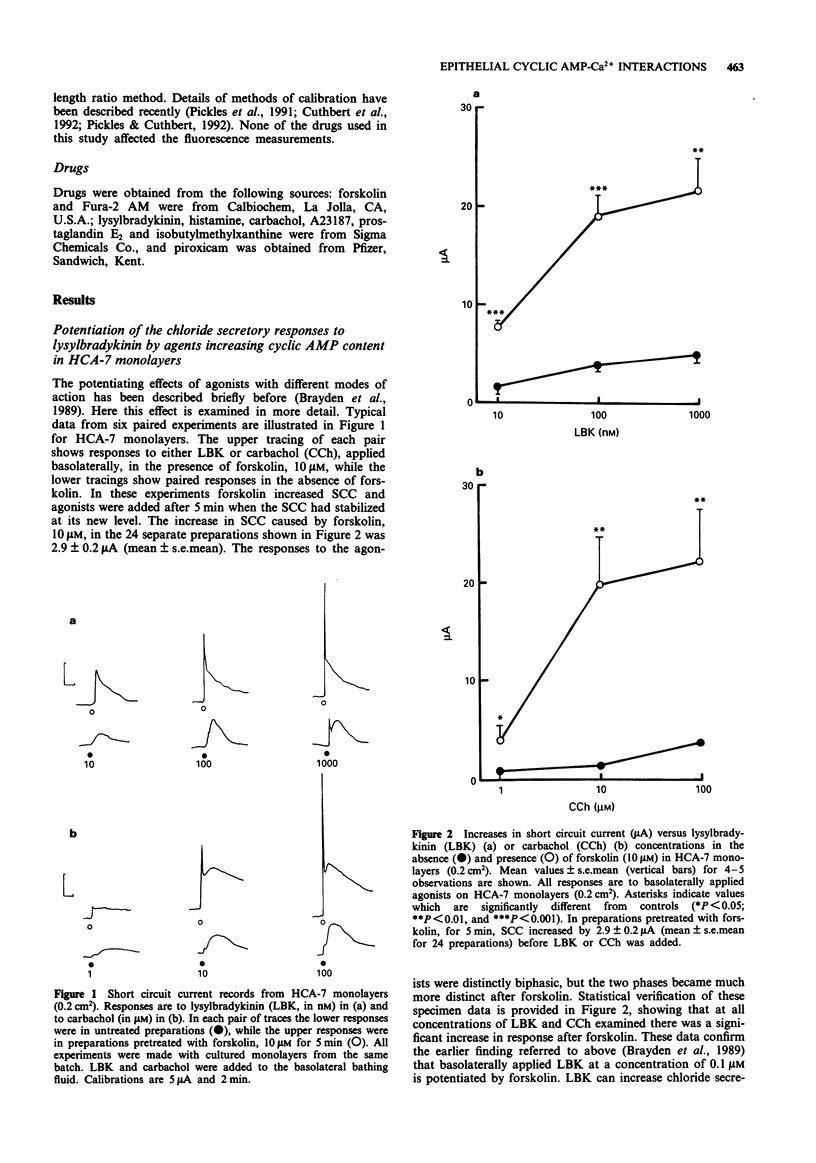
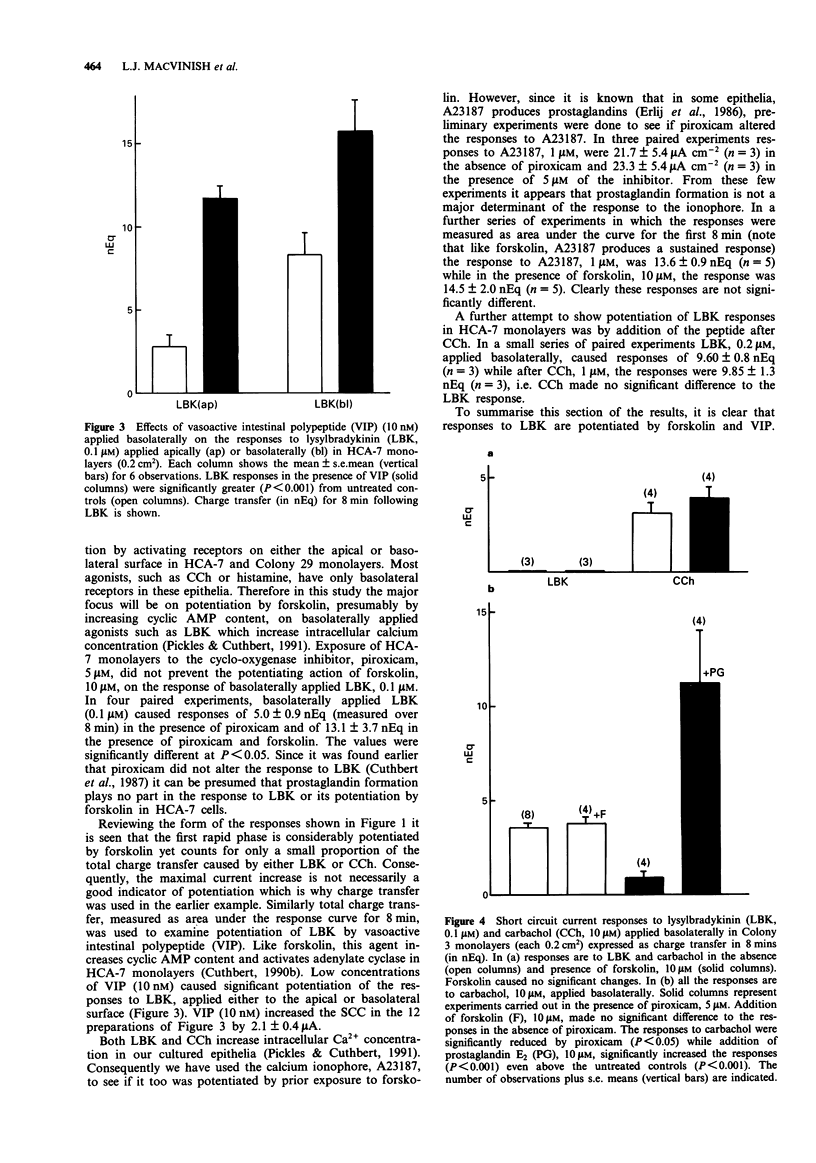
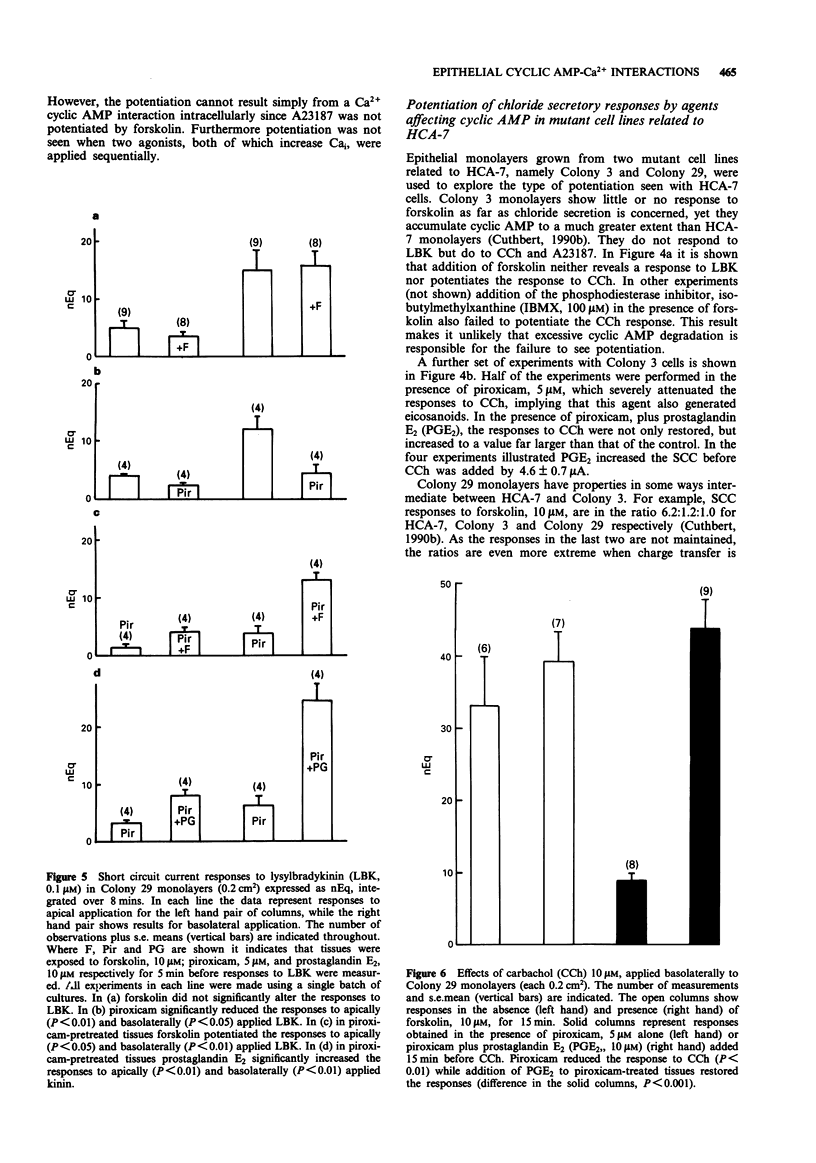
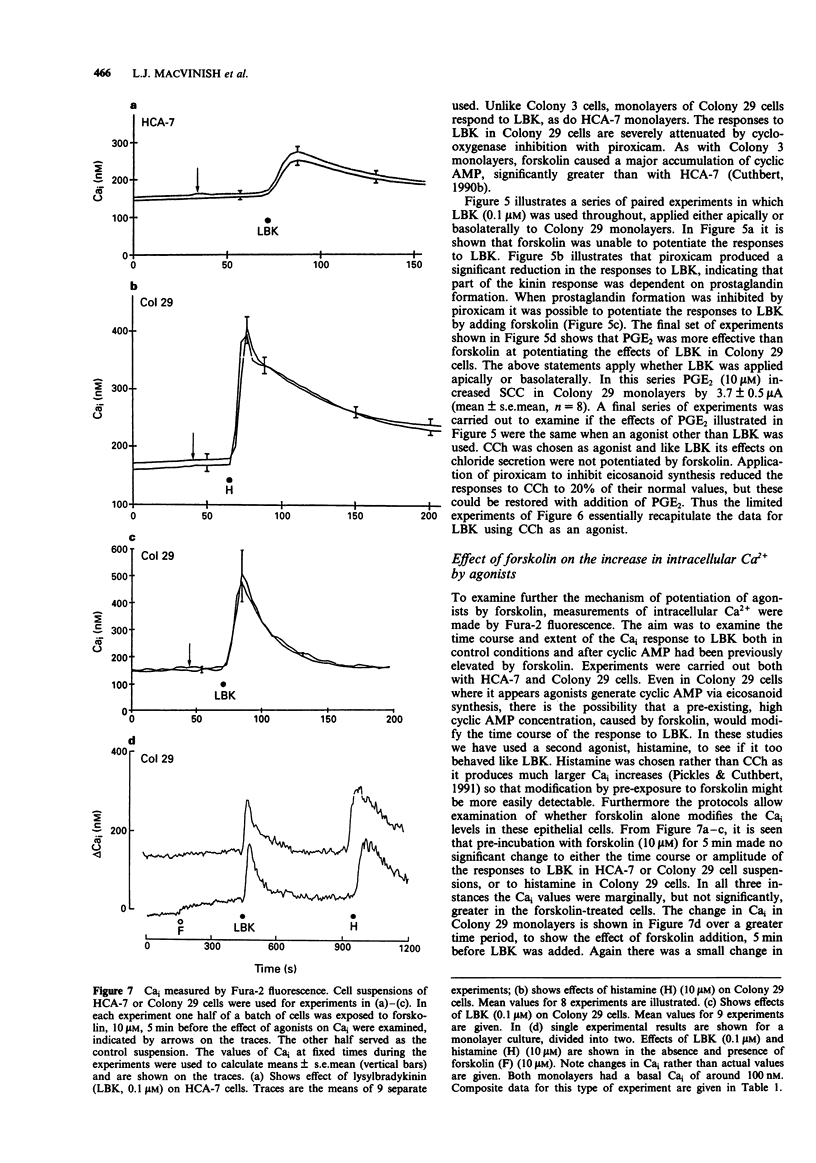
1. Chloride secretion in three types of cultured epithelial monolayers derived from a single human colonic adenocarcinoma was measured in terms of short circuit current. The three cell types were designated HCA-7, Colony 3 and Colony 29. 2. Responses of HCA-7 monolayers to basolaterally applied lysylbradykinin (LBK) (10-1000 nM) or carbachol (1-100 microM) were potentiated by pre-exposure to forskolin (10 microM) for 5 min. Forskolin itself increased short circuit current (SCC), so that the total response to forskolin and LBK or carbachol were non-additive. 3. Colony 3 cells did not respond to LBK on either face but did to carbachol on the basolateral side, while Colony 29 epithelia responded to LBK on both sides and to carbachol and histamine basolaterally. Unlike HCA-7 epithelia, responses in Colony 3 and Colony 29 epithelia were not potentiated by forskolin, but were attenuated by piroxicam. 4. In the presence of piroxicam, both forskolin and prostaglandin E2 were able to potentiate the action of both LBK and carbachol in Colony 29 epithelia. 5. LBK receptor activation in Colony 29 epithelia is transduced into an increase in intracellular Ca2+ and cyclic AMP, while in HCA-7 epithelia there is only an increase in intracellular Ca2+ (Cai). These conclusions are considered to apply to both apical and basolateral kinin receptors. 6. It is shown that forskolin has no effect on the elevation of Ca2+ by LBK in either HCA-7 or Colony 29 cells. 7. It is concluded that potentiation of agonist responses occurs when cyclic AMP is raised at the time that intracellular Ca2+ increases.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brayden D. J., Hanley M. R., Thastrup O., Cuthbert A. W. Thapsigargin, a new calcium-dependent epithelial anion secretagogue. Br J Pharmacol. 1989 Nov;98(3):809–816. doi: 10.1111/j.1476-5381.1989.tb14609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Egléme C., Greenwood H., Hickman M. E., Kirkland S. C., MacVinish L. J. Calcium- and cyclic AMP-dependent chloride secretion in human colonic epithelia. Br J Pharmacol. 1987 Jul;91(3):503–515. doi: 10.1111/j.1476-5381.1987.tb11243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Halushka P. V., Margolius H. S., Spayne J. A. Mediators of the secretory response to kinins. Br J Pharmacol. 1984 Jul;82(3):597–607. doi: 10.1111/j.1476-5381.1984.tb10798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., MacVinish L. J., Pickles R. J. Antagonism of kinin effects on epithelial by Hoe 140: apparently competitive and non-competitive interactions. Br J Pharmacol. 1992 Nov;107(3):797–802. doi: 10.1111/j.1476-5381.1992.tb14526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson K. E., Frizzell R. A., Sekar M. C. Activation of T84 cell chloride channels by carbachol involves a phosphoinositide-coupled muscarinic M3 receptor. Eur J Pharmacol. 1992 Apr 10;225(4):291–298. doi: 10.1016/0922-4106(92)90102-2. [DOI] [PubMed] [Google Scholar]
- Erlij D., Gersten L., Sterba G., Schoen H. F. Role of prostaglandin release in the response of tight epithelia to Ca2+ ionophores. Am J Physiol. 1986 Apr;250(4 Pt 1):C629–C636. doi: 10.1152/ajpcell.1986.250.4.C629. [DOI] [PubMed] [Google Scholar]
- Fischer H., Illek B., Negulescu P. A., Clauss W., Machen T. E. Carbachol-activated calcium entry into HT-29 cells is regulated by both membrane potential and cell volume. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1438–1442. doi: 10.1073/pnas.89.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Henderson R. M., Ashford M. L., MacVinish L. J., Cuthbert A. W. Chloride channels and anion fluxes in a human colonic epithelium (HCA-7). Br J Pharmacol. 1992 May;106(1):109–114. doi: 10.1111/j.1476-5381.1992.tb14301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. M., Cuthbert A. W. Activation of ion channels by lysylbradykinin in the HCA-7 colony 29 human adenocarcinoma cell line. Br J Pharmacol. 1993 Feb;108(2):479–483. doi: 10.1111/j.1476-5381.1993.tb12828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland S. C. Dome formation by a human colonic adenocarcinoma cell line (HCA-7). Cancer Res. 1985 Aug;45(8):3790–3795. [PubMed] [Google Scholar]
- MacVinish L. J., Reancharoen T., Cuthbert A. W. Kinin-induced chloride permeability changes in colony 29 epithelia estimated from 125I- efflux and MEQ fluorescence. Br J Pharmacol. 1993 Feb;108(2):469–478. doi: 10.1111/j.1476-5381.1993.tb12827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Kirk K. L., Frizzell R. A. Simultaneous analysis of cell Ca2+ and Ca2(+)-stimulated chloride conductance in colonic epithelial cells (HT-29). Cell Regul. 1990 Nov;1(12):951–963. doi: 10.1091/mbc.1.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulais M., Turner R. J. Beta-adrenergic upregulation of the Na(+)-K(+)-2Cl- cotransporter in rat parotid acinar cells. J Clin Invest. 1992 Apr;89(4):1142–1147. doi: 10.1172/JCI115695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewitt E. B., Hegde R. S., Haas M., Palfrey H. C. The regulation of Na/K/2Cl cotransport and bumetanide binding in avian erythrocytes by protein phosphorylation and dephosphorylation. Effects of kinase inhibitors and okadaic acid. J Biol Chem. 1990 Dec 5;265(34):20747–20756. [PubMed] [Google Scholar]
- Pickles R. J., Brayden D. J., Cuthbert A. W. Synchronous transporting activity in epithelial cells in relation to intracellular calcium concentration. Proc Biol Sci. 1991 Jul 22;245(1312):53–58. doi: 10.1098/rspb.1991.0087. [DOI] [PubMed] [Google Scholar]
- Pickles R. J., Cuthbert A. W. Failure of thapsigargin to alter ion transport in human sweat gland epithelia while intracellular Ca2+ concentration is raised. J Biol Chem. 1992 Jul 25;267(21):14818–14825. [PubMed] [Google Scholar]
- Pickles R. J., Cuthbert A. W. Relation of anion secretory activity to intracellular Ca2+ in response to lysylbradykinin and histamine in a cultured human colonic epithelium. Eur J Pharmacol. 1991 Jun 18;199(1):77–91. doi: 10.1016/0014-2999(91)90639-8. [DOI] [PubMed] [Google Scholar]
- Smith J. J., McCann J. D., Welsh M. J. Bradykinin stimulates airway epithelial Cl- secretion via two second messenger pathways. Am J Physiol. 1990 Jun;258(6 Pt 1):L369–L377. doi: 10.1152/ajplung.1990.258.6.L369. [DOI] [PubMed] [Google Scholar]


