Abstract
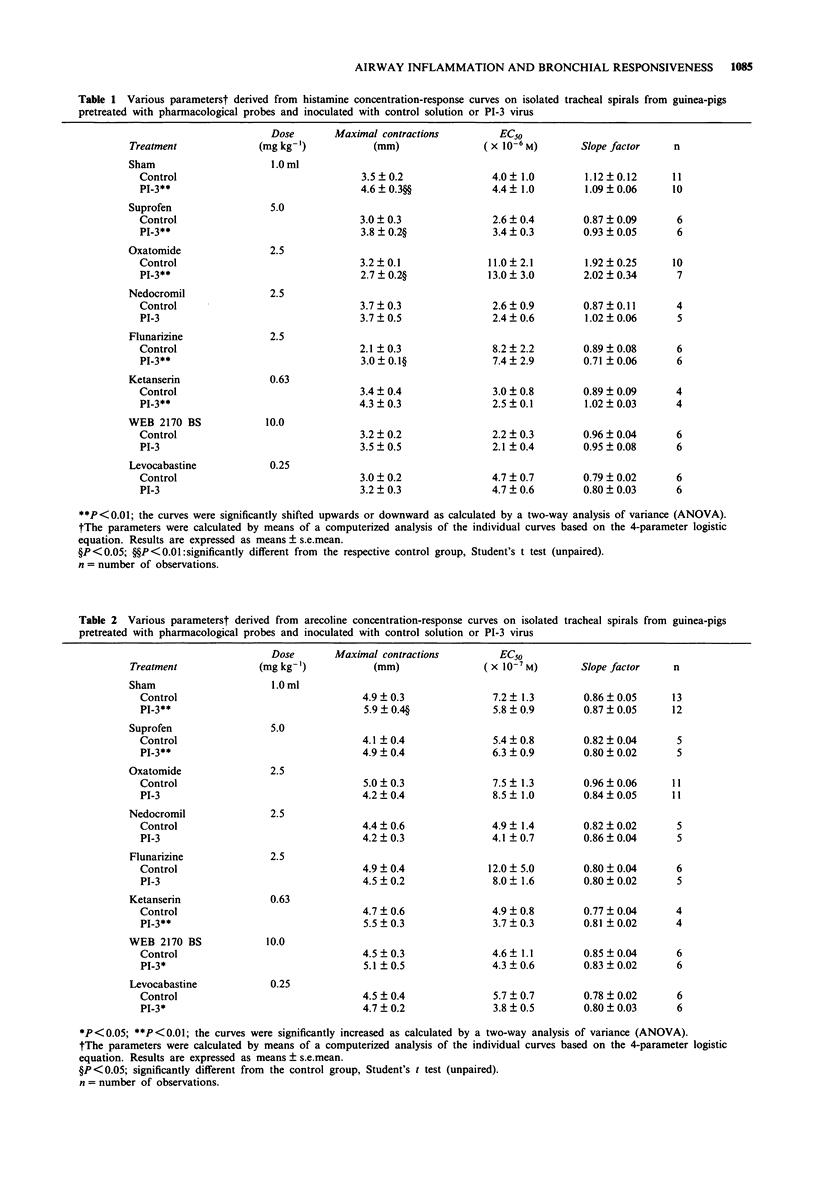
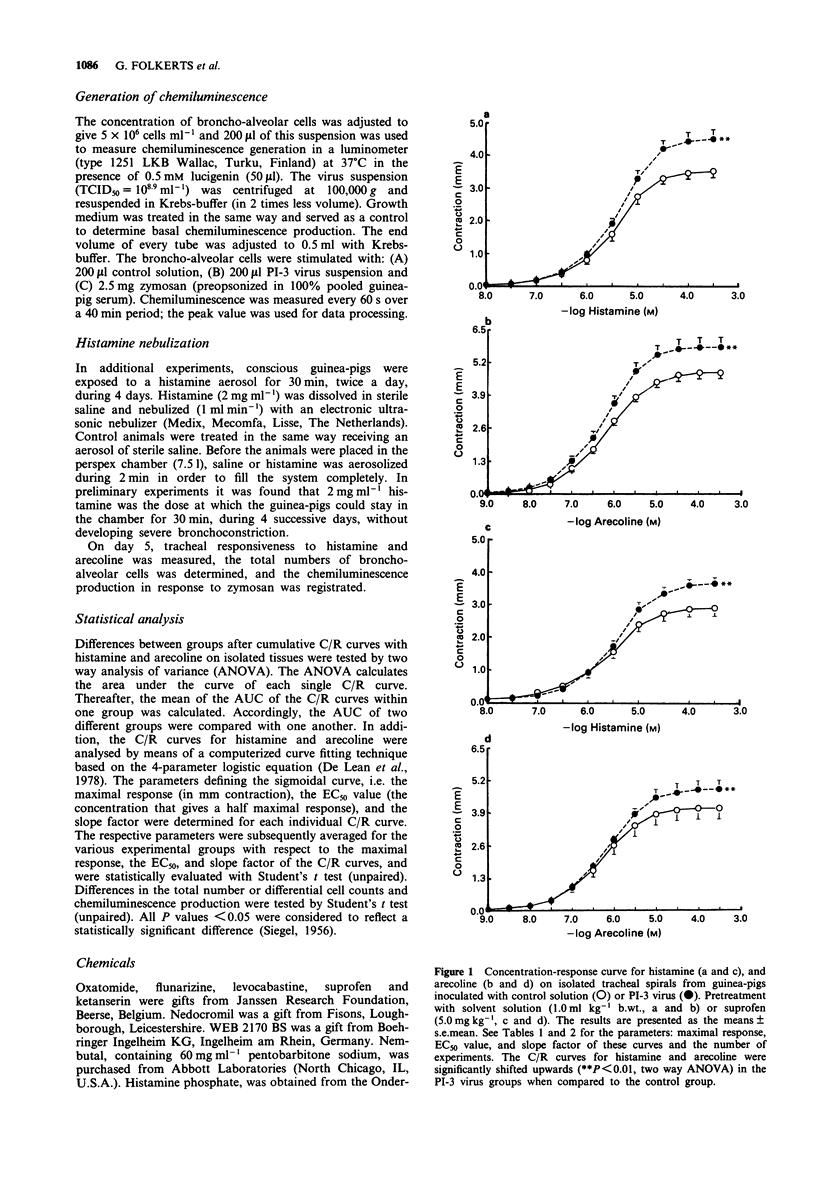
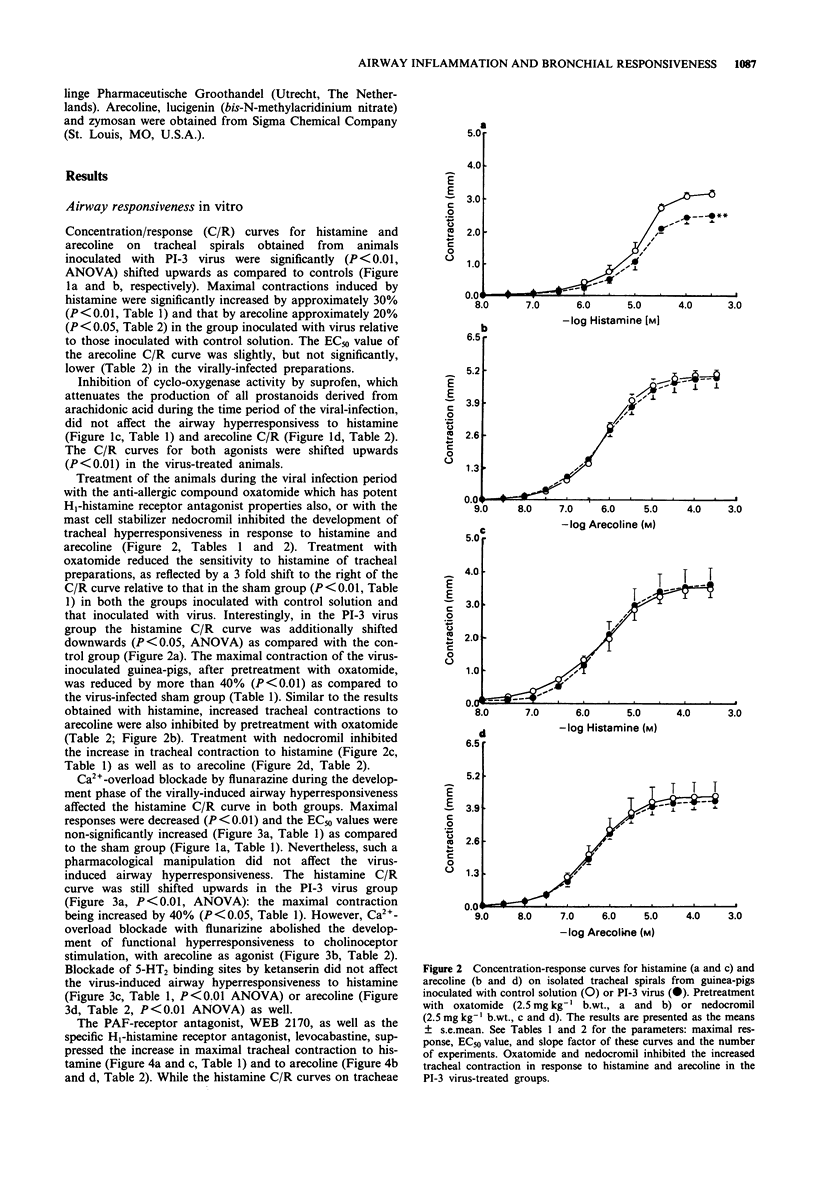
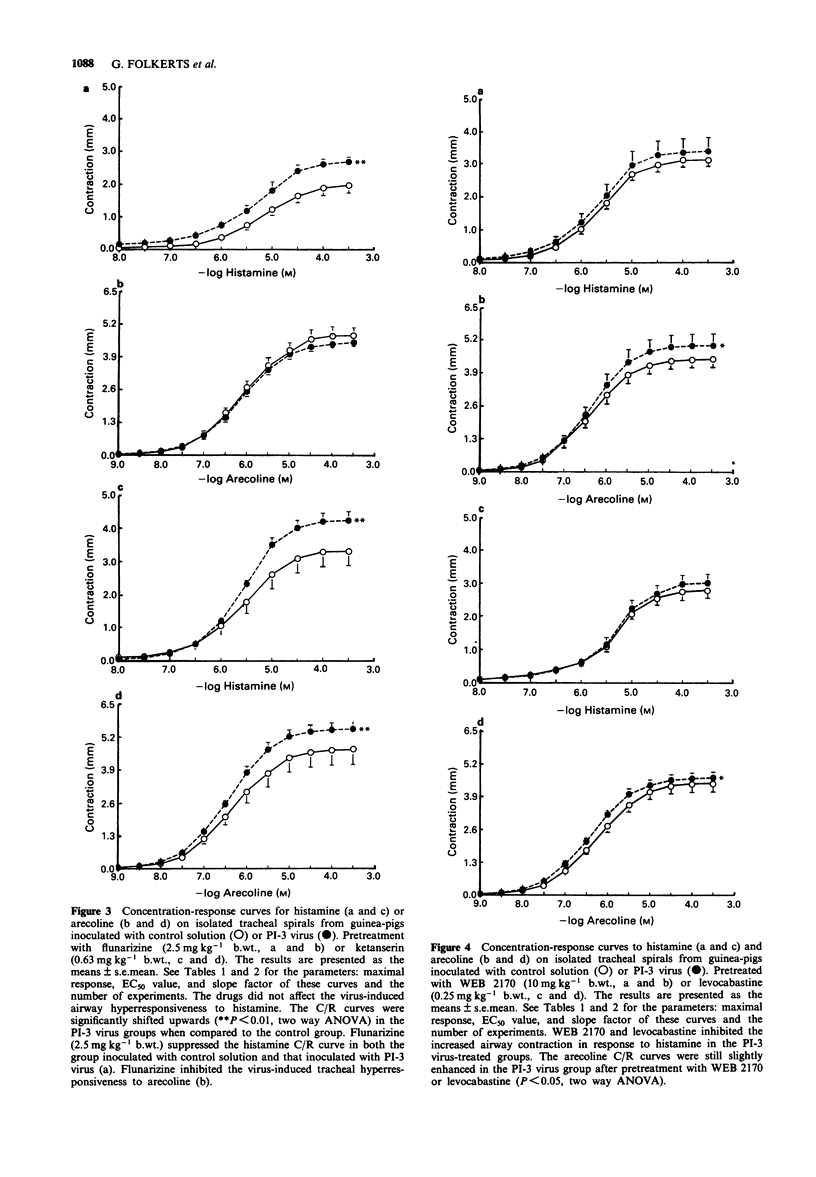
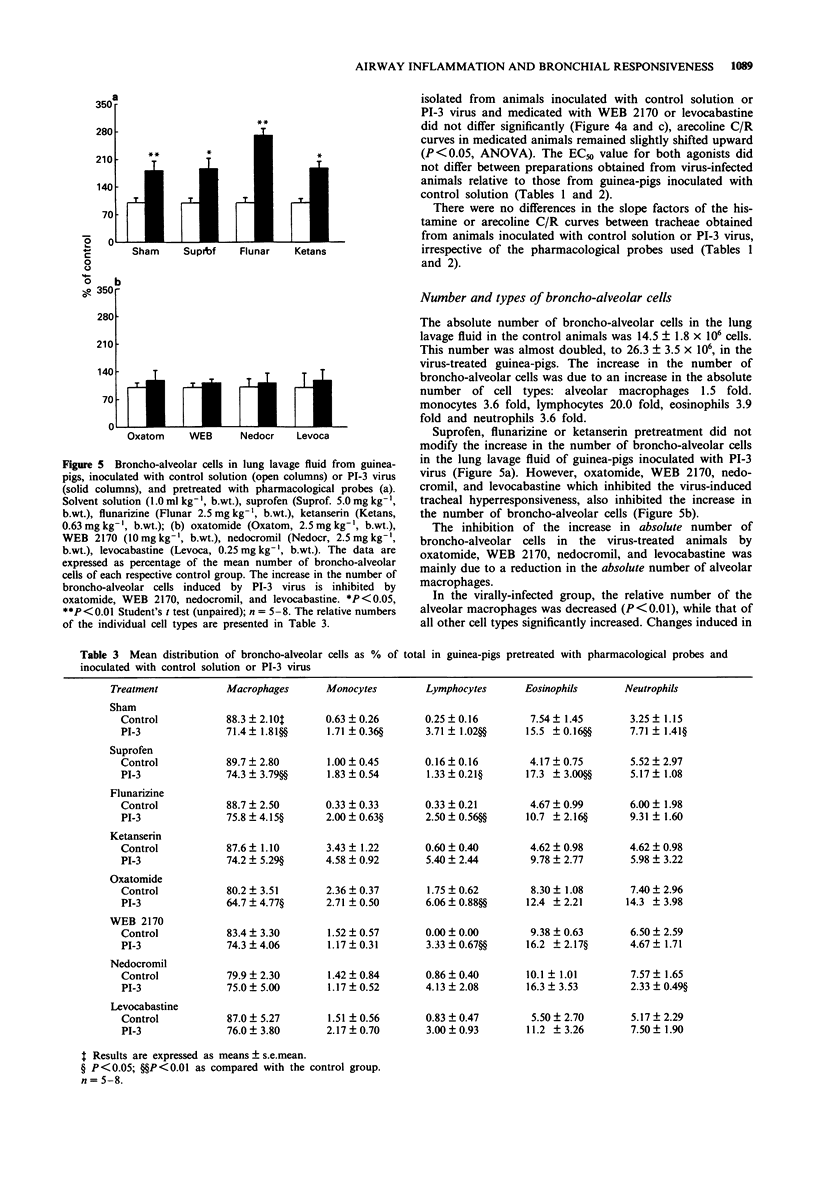
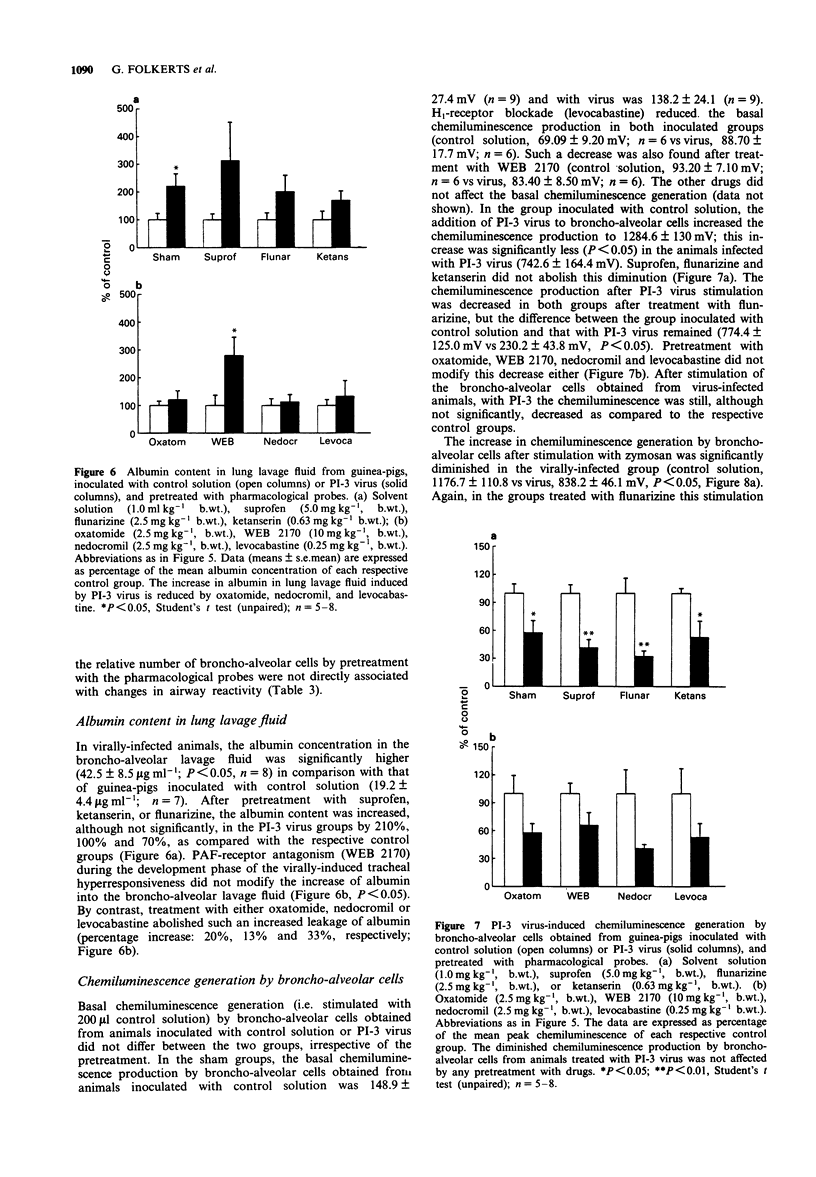
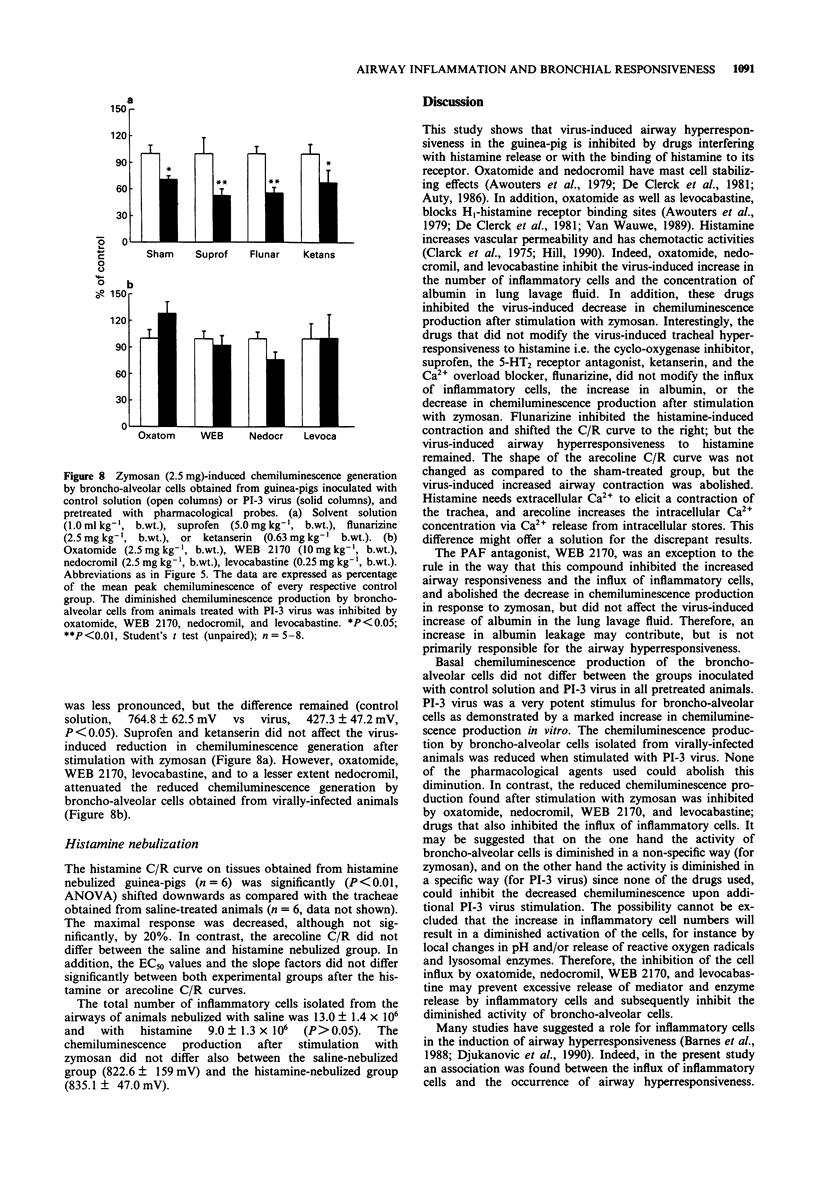
1. Guinea-pig tracheal contractions by histamine and by the cholinoceptor agonist, arecoline, are significantly enhanced (30% and 20%, respectively), 96 h after intra-tracheal inoculation with Parainfluenza-3 (PI-3) virus. 2. The airway hyperresponsiveness in animals inoculated with virus coincides with a significant increase in the number of broncho-alveolar cells (82%), and in the albumin concentration (121%) in lung lavage fluid, relative to values obtained in guinea-pigs challenged with control solution. 3. The chemiluminescence production by isolated broncho-alveolar cells, obtained from virus-infected guinea-pigs 96 h after inoculation stimulated with PI-3 virus in vitro, is significantly reduced by 42% relative to broncho-alveolar cells obtained from animals inoculated with control solution. This diminution was not specific for stimulation by PI-3 virus since the chemiluminescence production was also significantly reduced by 30% in response to zymosan. 4. Pretreatment of the guinea-pigs with the anti-allergic drugs, oxatomide (2.5 mg kg-1) or nedocromil (2.5 mg kg-1), or the specific H1-histamine receptor antagonist, levocabastine (0.25 mg kg-1), administered intra-peritoneally twice a day for five successive days, inhibits the virus-induced airway hyperresponsiveness, suppresses the influx of broncho-alveolar cells and increase in albumin content, and corrects the reduced chemiluminescence production by broncho-alveolar cells in response to zymosan. 5. In contrast, the cyclo-oxygenase inhibitor, suprofen (5.0 mg kg-1), the 5-HT2 receptor antagonist, ketanserin (0.63 mg kg-1), or the Ca2+ overload blocker, flunarizine (2.5 mg kg-1) do not modify the above mentioned processes. 6. The platelet-activating factor receptor antagonist, WEB 2170 (10 mg kg-1), reduces virus-induced airway hyperresponsiveness and influx of broncho-alveolar cells into the lungs but does not attenuate the increase of albumin in the bronchial lavage fluid. 7. Guinea-pigs nebulized with histamine, twice a day (30 min) during 4 successive days, do not demonstrate an increased airway responsiveness, but instead show tachyphylaxis in response to histamine in vitro. In addition, no influx of inflammatory cells is found in these animals. 8. These results suggest that histamine does not directly increase the responsiveness of the guinea-pig trachea; however, histamine may be involved in a cascade of events leading to airway hyperresponsiveness after a viral infection, a process that could be related to an influx and/or an activation of broncho-alveolar cells after PI-3 virus stimulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquilina A. T., Hall W. J., Douglas R. G., Jr, Utell M. J. Airway reactivity in subjects with viral upper respiratory tract infections: the effects of exercise and cold air. Am Rev Respir Dis. 1980 Jul;122(1):3–10. doi: 10.1164/arrd.1980.122.1.3. [DOI] [PubMed] [Google Scholar]
- Auty R. M. The clinical development of a new agent for the treatment of airway inflammation, nedocromil sodium (Tilade). Eur J Respir Dis Suppl. 1986;147:120–131. [PubMed] [Google Scholar]
- Barnes P. J., Belvisi M. G., Rogers D. F. Modulation of neurogenic inflammation: novel approaches to inflammatory disease. Trends Pharmacol Sci. 1990 May;11(5):185–189. doi: 10.1016/0165-6147(90)90112-l. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Chung K. F., Page C. P. Inflammatory mediators and asthma. Pharmacol Rev. 1988 Mar;40(1):49–84. [PubMed] [Google Scholar]
- Busse W. W. Respiratory infections: their role in airway responsiveness and the pathogenesis of asthma. J Allergy Clin Immunol. 1990 Apr;85(4):671–683. doi: 10.1016/0091-6749(90)90181-3. [DOI] [PubMed] [Google Scholar]
- Casale T. B., Wood D., Richerson H. B., Trapp S., Metzger W. J., Zavala D., Hunninghake G. W. Elevated bronchoalveolar lavage fluid histamine levels in allergic asthmatics are associated with methacholine bronchial hyperresponsiveness. J Clin Invest. 1987 Apr;79(4):1197–1203. doi: 10.1172/JCI112937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Gallin J. I., Kaplan A. P. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975 Dec 1;142(6):1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clerck F., Van Reempts J., Borgers M. Comparative effects of oxatomide on the release of histamine from rat peritoneal mast cells. Agents Actions. 1981 May;11(3):184–192. doi: 10.1007/BF01967612. [DOI] [PubMed] [Google Scholar]
- De Clerck F., Vermylen J., Reneman R. Effects of suprofen, an inhibitor of prostaglandin biosynthesis, on platelet function, plasma coagulation and fibrinolysis. I. In vitro experiments. Arch Int Pharmacodyn Ther. 1975 Aug;216(2):263–279. [PubMed] [Google Scholar]
- De Clerck F., Xhonneux B., Leysen J., Janssen P. A. Evidence for functional 5-HT2 receptor sites on human blood platelets. Biochem Pharmacol. 1984 Sep 1;33(17):2807–2811. doi: 10.1016/0006-2952(84)90699-3. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Doumas B. T., Watson W. A., Biggs H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971 Jan;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Empey D. W., Laitinen L. A., Jacobs L., Gold W. M., Nadel J. A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976 Feb;113(2):131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Engels F., Nijkamp F. P. Endotoxin-induced hyperreactivity of the guinea-pig isolated trachea coincides with decreased prostaglandin E2 production by the epithelial layer. Br J Pharmacol. 1989 Feb;96(2):388–394. doi: 10.1111/j.1476-5381.1989.tb11829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerts G., Henricks P. A., Slootweg P. J., Nijkamp F. P. Endotoxin-induced inflammation and injury of the guinea pig respiratory airways cause bronchial hyporeactivity. Am Rev Respir Dis. 1988 Jun;137(6):1441–1448. doi: 10.1164/ajrccm/137.6.1441. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Van Esch B., Janssen M., Nijkamp F. P. Virus-induced airway hyperresponsiveness in guinea pigs in vivo: study of broncho-alveolar cell number and activity. Eur J Pharmacol. 1992 Dec 1;228(4):219–227. doi: 10.1016/0926-6917(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Verheyen A., Janssen M., Nijkamp F. P. Virus-induced airway hyperresponsiveness in the guinea pig can be transferred by bronchoalveolar cells. J Allergy Clin Immunol. 1992 Sep;90(3 Pt 1):364–372. doi: 10.1016/s0091-6749(05)80016-8. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Verheyen A., Nijkamp F. P. Viral infection in guinea pigs induces a sustained non-specific airway hyperresponsiveness and morphological changes of the respiratory tract. Eur J Pharmacol. 1992 Sep 1;228(2-3):121–130. doi: 10.1016/0926-6917(92)90021-4. [DOI] [PubMed] [Google Scholar]
- Graziano F. M., Tilton R., Hirth T., Segaloff D., Mullins T., Dick E., Buckner C. K., Busse W. W. The effect of parainfluenza 3 infection on guinea pig basophil and lung mast cell histamine release. Am Rev Respir Dis. 1989 Mar;139(3):715–720. doi: 10.1164/ajrccm/139.3.715. [DOI] [PubMed] [Google Scholar]
- Hall C. B., Douglas R. G., Jr, Simons R. L., Geiman J. M. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J Pediatr. 1978 Jul;93(1):28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- Heuer H. O., Casals-Stenzel J., Muacevic G., Weber K. H. Pharmacologic activity of bepafant (WEB 2170), a new and selective hetrazepinoic antagonist of platelet activating factor. J Pharmacol Exp Ther. 1990 Dec;255(3):962–968. [PubMed] [Google Scholar]
- Hill S. J. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990 Mar;42(1):45–83. [PubMed] [Google Scholar]
- Jacoby D. B., Tamaoki J., Borson D. B., Nadel J. A. Influenza infection causes airway hyperresponsiveness by decreasing enkephalinase. J Appl Physiol (1985) 1988 Jun;64(6):2653–2658. doi: 10.1152/jappl.1988.64.6.2653. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Kava T. Bronchial reactivity following uncomplicated influenza A infection in healthy subjects and in asthmatic patients. Eur J Respir Dis Suppl. 1980;106:51–58. [PubMed] [Google Scholar]
- Lellouch-Tubiana A., Lefort J., Simon M. T., Pfister A., Vargaftig B. B. Eosinophil recruitment into guinea pig lungs after PAF-acether and allergen administration. Modulation by prostacyclin, platelet depletion, and selective antagonists. Am Rev Respir Dis. 1988 Apr;137(4):948–954. doi: 10.1164/ajrccm/137.4.948. [DOI] [PubMed] [Google Scholar]
- Little J. W., Hall W. J., Douglas R. G., Jr, Mudholkar G. S., Speers D. M., Patel K. Airway hyperreactivity and peripheral airway dysfunction in influenza A infection. Am Rev Respir Dis. 1978 Aug;118(2):295–303. doi: 10.1164/arrd.1978.118.2.295. [DOI] [PubMed] [Google Scholar]
- Maggi C. A. The pharmacology of the efferent function of sensory nerves. J Auton Pharmacol. 1991 Jun;11(3):173–208. doi: 10.1111/j.1474-8673.1991.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Mannaioni P. F., Masini E. The release of histamine by free radicals. Free Radic Biol Med. 1988;5(3):177–197. doi: 10.1016/0891-5849(88)90080-9. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Ellis E. F., Hoffman L. S., Lybass T. G., Eller J. J., Fulginiti V. A. The association of viral and bacterial respiratory infections with exacerbations of wheezing in young asthmatic children. J Pediatr. 1973 Apr;82(4):578–590. doi: 10.1016/S0022-3476(73)80582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor T. E., Dick E. C., DeMeo A. N., Ouellette J. J., Cohen M., Reed C. E. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974 Jan 21;227(3):292–298. [PubMed] [Google Scholar]
- O'Donnell S. R., Barnett C. J. Microvascular leakage to platelet activating factor in guinea-pig trachea and bronchi. Eur J Pharmacol. 1987 Jun 26;138(3):385–396. doi: 10.1016/0014-2999(87)90477-8. [DOI] [PubMed] [Google Scholar]
- Ogunbiyi P. O., Black W. D., Eyre P. Parainfluenza-3 virus-induced enhancement of histamine release from calf lung mast cells--effect of levamisole. J Vet Pharmacol Ther. 1988 Dec;11(4):338–344. doi: 10.1111/j.1365-2885.1988.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Okuda Y., Tsuyuguchi I., Yamatodani A. Histamine release from human leukocytes by platelet-activating factor. Int Arch Allergy Appl Immunol. 1988;85(3):341–345. doi: 10.1159/000234529. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Rafferty P. Antihistamines in the treatment of clinical asthma. J Allergy Clin Immunol. 1990 Oct;86(4 Pt 2):647–650. doi: 10.1016/s0091-6749(05)80231-3. [DOI] [PubMed] [Google Scholar]
- Saban R., Dick E. C., Fishleder R. I., Buckner C. K. Enhancement by parainfluenza 3 infection of contractile responses to substance P and capsaicin in airway smooth muscle from the guinea pig. Am Rev Respir Dis. 1987 Sep;136(3):586–591. doi: 10.1164/ajrccm/136.3.586. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Vanbenthuysen K. M., Shasby D. M., McMurtry I. F., Repine J. E. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Todd P. A., Benfield P. Flunarizine. A reappraisal of its pharmacological properties and therapeutic use in neurological disorders. Drugs. 1989 Oct;38(4):481–499. doi: 10.2165/00003495-198938040-00002. [DOI] [PubMed] [Google Scholar]
- Tosi M. F., Stark J. M., Hamedani A., Smith C. W., Gruenert D. C., Huang Y. T. Intercellular adhesion molecule-1 (ICAM-1)-dependent and ICAM-1-independent adhesive interactions between polymorphonuclear leukocytes and human airway epithelial cells infected with parainfluenza virus type 2. J Immunol. 1992 Nov 15;149(10):3345–3349. [PubMed] [Google Scholar]
- Wardlaw A. J., Dunnette S., Gleich G. J., Collins J. V., Kay A. B. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988 Jan;137(1):62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Wong D. T., Sun M., Middleton E., Jr, Vaughan R. S., Ogra P. L. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981 Oct 8;305(15):841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]


