Abstract
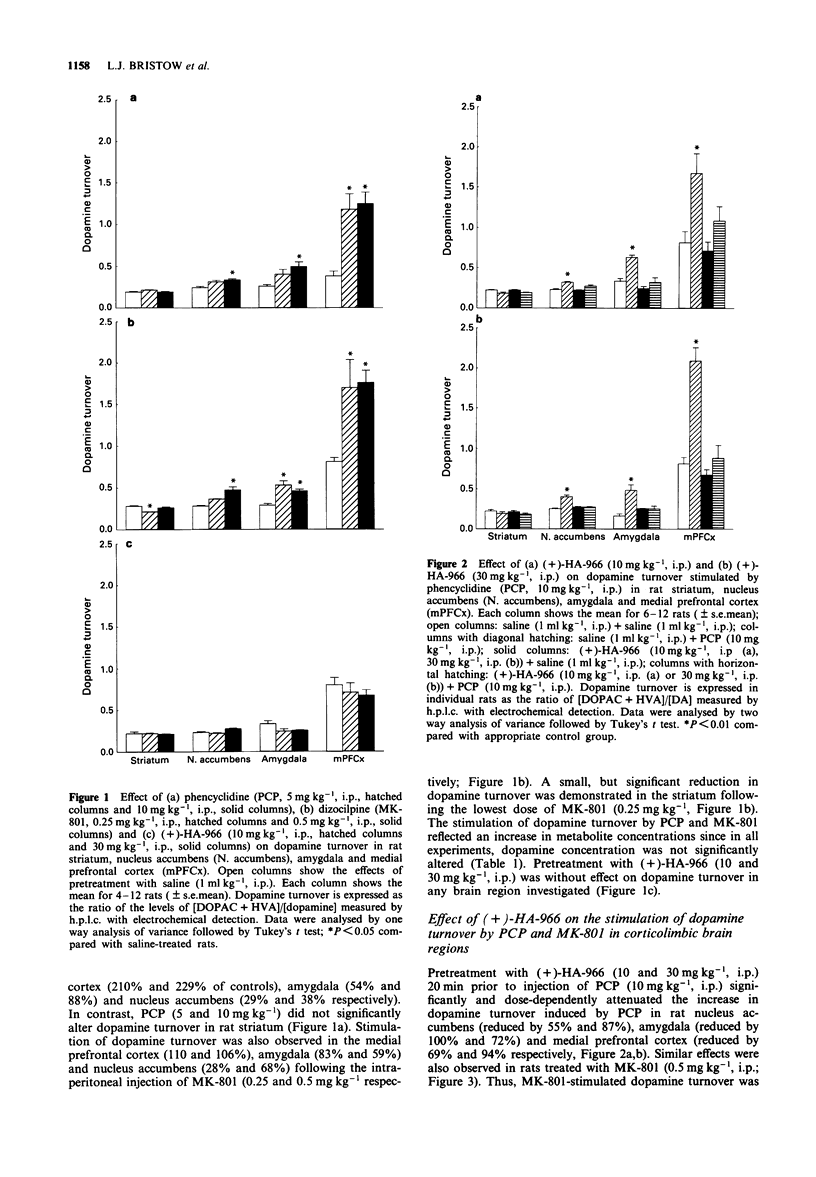
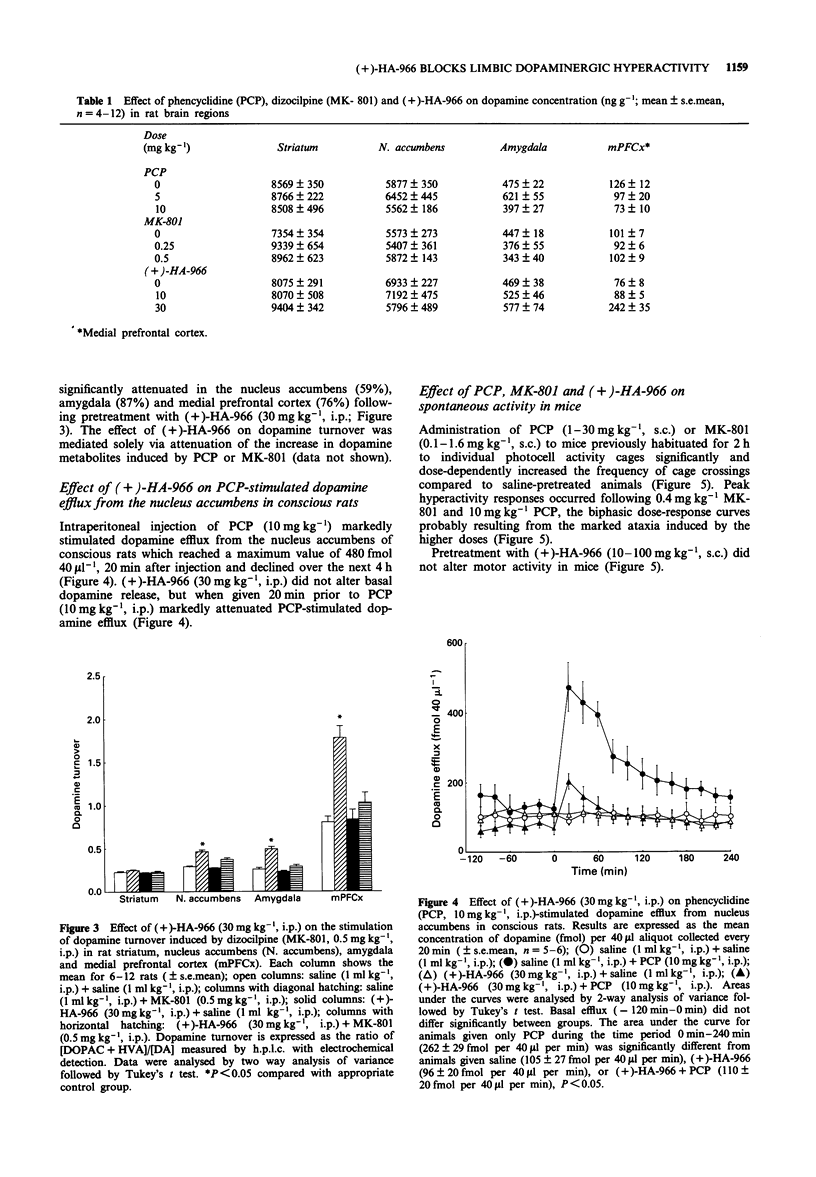
1. The effects of the glycine/N-methyl-D-aspartate (NMDA) receptor antagonist, R-(+)-HA-966 on the neurochemical and behavioural responses to phencyclidine (PCP) and dizocilpine (MK-801) have been determined in rodents. 2. In rats, pretreatment with PCP (5 and 10 mg kg-1) or MK-801 (0.25 and 0.5 mg kg-1) dose-dependently stimulated dopamine turnover in nucleus accumbens, amygdala and medial prefrontal cortex, but had no effect in striatum. In contrast, pretreatment with (+)-HA-966 (10 and 30 mg kg-1) did not affect dopamine turnover in any brain region investigated. 3. Pretreatment with (+)-HA-966 (10 and 30 mg kg-1) significantly antagonized the stimulation of dopamine turnover induced by both PCP (10 mg kg-1) and MK-801 (0.5 mg kg-1) in rat nucleus accumbens, amygdala and medial prefrontal cortex. 4. Intracerebral dialysis studies in conscious rats demonstrated that systemic injection of PCP (10 mg kg-1) markedly stimulated dopamine release from the nucleus accumbens, an effect that was abolished by pretreatment with (+)-HA-966 (30 mg kg-1). 5. Pretreatment with PCP (3-30 mg kg-1) or MK-801 (0.1-1.6 mg kg-1) significantly increased locomotor activity in mice. In contrast, subcutaneous injection of (+)-HA-966 (10-100 mg kg-1) failed to stimulate activity. 6. Pretreatment with (+)-HA-966 (10 and 30 mg kg-1) dose-dependently antagonized both PCP (10 mg kg-1) and MK-801 (0.4 mg kg-1) induced hyperactivity in mice.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers M. B., Jr, Hoffman F. J., Jr Homovanillic acid in rat caudate and prefrontal cortex following phencyclidine and amphetamine. Psychopharmacology (Berl) 1984;84(1):136–137. doi: 10.1007/BF00432043. [DOI] [PubMed] [Google Scholar]
- Contreras P. C. D-serine antagonized phencyclidine- and MK-801-induced stereotyped behavior and ataxia. Neuropharmacology. 1990 Mar;29(3):291–293. doi: 10.1016/0028-3908(90)90015-j. [DOI] [PubMed] [Google Scholar]
- Deutch A. Y., Tam S. Y., Freeman A. S., Bowers M. B., Jr, Roth R. H. Mesolimbic and mesocortical dopamine activation induced by phencyclidine: contrasting pattern to striatal response. Eur J Pharmacol. 1987 Feb 24;134(3):257–264. doi: 10.1016/0014-2999(87)90356-6. [DOI] [PubMed] [Google Scholar]
- Donzanti B. A., Uretsky N. J. Antagonism of the hypermotility response induced by excitatory amino acids in the rat nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jan;325(1):1–7. doi: 10.1007/BF00507046. [DOI] [PubMed] [Google Scholar]
- Donzanti B. A., Uretsky N. J. Effects of excitatory amino acids on locomotor activity after bilateral microinjection into the rat nucleus accumbens: possible dependence on dopaminergic mechanisms. Neuropharmacology. 1983 Aug;22(8):971–981. doi: 10.1016/0028-3908(83)90213-7. [DOI] [PubMed] [Google Scholar]
- French E. D. Competitive NMDA receptor antagonists attenuate phencyclidine-induced excitations of A10 dopamine neurons. Eur J Pharmacol. 1992 Jun 24;217(1):1–7. doi: 10.1016/0014-2999(92)90503-v. [DOI] [PubMed] [Google Scholar]
- French E. D., Ferkany J., Abreu M., Levenson S. Effects of competitive N-methyl-D-aspartate antagonists on midbrain dopamine neurons: an electrophysiological and behavioral comparison to phencyclidine. Neuropharmacology. 1991 Oct;30(10):1039–1046. doi: 10.1016/0028-3908(91)90131-t. [DOI] [PubMed] [Google Scholar]
- French E. D., Pilapil C., Quirion R. Phencyclidine binding sites in the nucleus accumbens and phencyclidine-induced hyperactivity are decreased following lesions of the mesolimbic dopamine system. Eur J Pharmacol. 1985 Oct 8;116(1-2):1–9. doi: 10.1016/0014-2999(85)90178-5. [DOI] [PubMed] [Google Scholar]
- French E. D., Vantini G. Phencyclidine-induced locomotor activity in the rat is blocked by 6-hydroxydopamine lesion of the nucleus accumbens: comparisons to other psychomotor stimulants. Psychopharmacology (Berl) 1984;82(1-2):83–88. doi: 10.1007/BF00426386. [DOI] [PubMed] [Google Scholar]
- Hamilton M. H., De Belleroche J. S., Gardiner I. M., Herberg L. J. Stimulatory effect of N-methyl aspartate on locomotor activity and transmitter release from rat nucleus accumbens. Pharmacol Biochem Behav. 1986 Nov;25(5):943–948. doi: 10.1016/0091-3057(86)90067-5. [DOI] [PubMed] [Google Scholar]
- Hayes B. A., Balster R. L. Anticonvulsant properties of phencyclidine-like drugs in mice. Eur J Pharmacol. 1985 Oct 29;117(1):121–125. doi: 10.1016/0014-2999(85)90480-7. [DOI] [PubMed] [Google Scholar]
- Hutson P. H., Bristow L. J., Thorn L., Tricklebank M. D. R-(+)-HA-966, a glycine/NMDA receptor antagonist, selectively blocks the activation of the mesolimbic dopamine system by amphetamine. Br J Pharmacol. 1991 Aug;103(4):2037–2044. doi: 10.1111/j.1476-5381.1991.tb12372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson P. H., Suman-Chauhan N. Activation of postsynaptic striatal dopamine receptors, monitored by efflux of cAMP in vivo. Neuropharmacology. 1990 Nov;29(11):1011–1016. doi: 10.1016/0028-3908(90)90106-2. [DOI] [PubMed] [Google Scholar]
- Javitt D. C., Zukin S. R. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991 Oct;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johnson K. M. Phencyclidine: behavioral and biochemical evidence supporting a role for dopamine. Fed Proc. 1983 Jun;42(9):2579–2583. [PubMed] [Google Scholar]
- Jones S. M., Snell L. D., Johnson K. M. Inhibition by phencyclidine of excitatory amino acid-stimulated release of neurotransmitter in the nucleus accumbens. Neuropharmacology. 1987 Feb-Mar;26(2-3):173–179. doi: 10.1016/0028-3908(87)90206-1. [DOI] [PubMed] [Google Scholar]
- Kabuto H., Yokoi I., Mizukawa K., Mori A. Effects of an N-methyl-D-aspartate receptor agonist and its antagonist CPP on the levels of dopamine and serotonin metabolites in rat striatum collected in vivo by using a brain dialysis technique. Neurochem Res. 1989 Nov;14(11):1075–1080. doi: 10.1007/BF00965613. [DOI] [PubMed] [Google Scholar]
- Largent B. L., Gundlach A. L., Snyder S. H. Pharmacological and autoradiographic discrimination of sigma and phencyclidine receptor binding sites in brain with (+)-[3H]SKF 10,047, (+)-[3H]-3-[3-hydroxyphenyl]-N-(1-propyl)piperidine and [3H]-1-[1-(2-thienyl)cyclohexyl]piperidine. J Pharmacol Exp Ther. 1986 Aug;238(2):739–748. [PubMed] [Google Scholar]
- Largent B. L., Gundlach A. L., Snyder S. H. Psychotomimetic opiate receptors labeled and visualized with (+)-[3H]3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4983–4987. doi: 10.1073/pnas.81.15.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent B. L., Wikström H., Snowman A. M., Snyder S. H. Novel antipsychotic drugs share high affinity for sigma receptors. Eur J Pharmacol. 1988 Oct 18;155(3):345–347. doi: 10.1016/0014-2999(88)90527-4. [DOI] [PubMed] [Google Scholar]
- Lodge D., Johnson K. M. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol Sci. 1990 Feb;11(2):81–86. doi: 10.1016/0165-6147(90)90323-z. [DOI] [PubMed] [Google Scholar]
- Maurice T., Vignon J., Kamenka J. M., Chicheportiche R. Differential interaction of phencyclidine-like drugs with the dopamine uptake complex in vivo. J Neurochem. 1991 Feb;56(2):553–559. doi: 10.1111/j.1471-4159.1991.tb08185.x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn L. G., Kerchner G. A., Kalra V., Zimmerman D. M., Leander J. D. Phencyclidine receptors in rat brain cortex. Biochem Pharmacol. 1984 Nov 15;33(22):3529–3535. doi: 10.1016/0006-2952(84)90133-3. [DOI] [PubMed] [Google Scholar]
- O'Neill K. A., Carelli R. M., Jarvis M. F., Liebman J. M. Hyperactivity induced by N-methyl-d-aspartate injections into nucleus accumbens: lack of evidence for mediation by dopaminergic neurons. Pharmacol Biochem Behav. 1989 Dec;34(4):739–745. doi: 10.1016/0091-3057(89)90268-2. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L., Swerdlow N. R., Koob G. F. Nucleus accumbens NMDA antagonist decreases locomotor activity produced by cocaine, heroin or accumbens dopamine, but not caffeine. Pharmacol Biochem Behav. 1991 Dec;40(4):841–845. doi: 10.1016/0091-3057(91)90095-j. [DOI] [PubMed] [Google Scholar]
- Pycock C. J., Dawbarn D. Acute motor effects of N-methyl-D-aspartic acid and kainic acid applied focally to mesencephalic dopamine cell body regions in the rat. Neurosci Lett. 1980 May 15;18(1):85–90. doi: 10.1016/0304-3940(80)90217-7. [DOI] [PubMed] [Google Scholar]
- Quirion R., Hammer R. P., Jr, Herkenham M., Pert C. B. Phencyclidine (angel dust)/sigma "opiate" receptor: visualization by tritium-sensitive film. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5881–5885. doi: 10.1073/pnas.78.9.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom R. W., Deschenes N. L. Glycine modulation of NMDA-evoked release of [3H]acetylcholine and [3H]dopamine from rat striatal slices. Neurosci Lett. 1989 Jan 30;96(3):323–328. doi: 10.1016/0304-3940(89)90399-6. [DOI] [PubMed] [Google Scholar]
- Rao T. S., Kim H. S., Lehmann J., Martin L. L., Wood P. L. Differential effects of phencyclidine (PCP) and ketamine on mesocortical and mesostriatal dopamine release in vivo. Life Sci. 1989;45(12):1065–1072. doi: 10.1016/0024-3205(89)90163-x. [DOI] [PubMed] [Google Scholar]
- Rao T. S., Kim H. S., Lehmann J., Martin L. L., Wood P. L. Selective activation of dopaminergic pathways in the mesocortex by compounds that act at the phencyclidine (PCP) binding site: tentative evidence for PCP recognition sites not coupled to N-methyl-D-aspartate (NMDA) receptors. Neuropharmacology. 1990 Mar;29(3):225–230. doi: 10.1016/0028-3908(90)90005-c. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Reid A. A., Monn J. A., Jacobson A. E., Rice K. C. The psychotomimetic drug phencyclidine labels two high affinity binding sites in guinea pig brain: evidence for N-methyl-D-aspartate-coupled and dopamine reuptake carrier-associated phencyclidine binding sites. Mol Pharmacol. 1989 Dec;36(6):887–896. [PubMed] [Google Scholar]
- Singh L., Donald A. E., Foster A. C., Hutson P. H., Iversen L. L., Iversen S. D., Kemp J. A., Leeson P. D., Marshall G. R., Oles R. J. Enantiomers of HA-966 (3-amino-1-hydroxypyrrolid-2-one) exhibit distinct central nervous system effects: (+)-HA-966 is a selective glycine/N-methyl-D-aspartate receptor antagonist, but (-)-HA-966 is a potent gamma-butyrolactone-like sedative. Proc Natl Acad Sci U S A. 1990 Jan;87(1):347–351. doi: 10.1073/pnas.87.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Menzies R., Tricklebank M. D. The discriminative stimulus properties of (+)-HA-966, an antagonist at the glycine/N-methyl-D-aspartate receptor. Eur J Pharmacol. 1990 Sep 4;186(1):129–132. doi: 10.1016/0014-2999(90)94069-a. [DOI] [PubMed] [Google Scholar]
- Singh L., Wong E. H., Kesingland A. C., Tricklebank M. D. Evidence against an involvement of the haloperidol-sensitive sigma recognition site in the discriminative stimulus properties of (+)-N-allylnormetazocine ((+)-SKF 10,047). Br J Pharmacol. 1990 Jan;99(1):145–151. doi: 10.1111/j.1476-5381.1990.tb14668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell L. D., Mueller Z. L., Gannon R. L., Silverman P. B., Johnson K. M. A comparison between classes of drugs having phencyclidine-like behavioral properties on dopamine efflux in vitro and dopamine metabolism in vivo. J Pharmacol Exp Ther. 1984 Nov;231(2):261–269. [PubMed] [Google Scholar]
- Snyder S. H. Phencyclidine. Nature. 1980 Jun 5;285(5764):355–356. doi: 10.1038/285355a0. [DOI] [PubMed] [Google Scholar]
- Su T. P. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther. 1982 Nov;223(2):284–290. [PubMed] [Google Scholar]
- Tam S. W., Cook L. Sigma opiates and certain antipsychotic drugs mutually inhibit (+)-[3H] SKF 10,047 and [3H]haloperidol binding in guinea pig brain membranes. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5618–5621. doi: 10.1073/pnas.81.17.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanii Y., Nishikawa T., Hashimoto A., Takahashi K. Stereoselective inhibition by D- and L-alanine of phencyclidine-induced locomotor stimulation in the rat. Brain Res. 1991 Nov 1;563(1-2):281–284. doi: 10.1016/0006-8993(91)91546-d. [DOI] [PubMed] [Google Scholar]
- Tricklebank M. D., Singh L., Oles R. J., Preston C., Iversen S. D. The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol. 1989 Aug 11;167(1):127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- Tricklebank M. D., Singh L., Oles R. J., Wong E. H., Iversen S. D. A role for receptors of N-methyl-D-aspartic acid in the discriminative stimulus properties of phencyclidine. Eur J Pharmacol. 1987 Sep 23;141(3):497–501. doi: 10.1016/0014-2999(87)90573-5. [DOI] [PubMed] [Google Scholar]
- Vickroy T. W., Johnson K. M. Similar dopamine-releasing effects of phencyclidine and nonamphetamine stimulants in striatal slices. J Pharmacol Exp Ther. 1982 Dec;223(3):669–674. [PubMed] [Google Scholar]
- Vignon J., Pinet V., Cerruti C., Kamenka J. M., Chicheportiche R. [3H]N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine ([3H]BTCP): a new phencyclidine analog selective for the dopamine uptake complex. Eur J Pharmacol. 1988 Apr 13;148(3):427–436. doi: 10.1016/0014-2999(88)90122-7. [DOI] [PubMed] [Google Scholar]
- Weber E., Sonders M., Quarum M., McLean S., Pou S., Keana J. F. 1,3-Di(2-[5-3H]tolyl)guanidine: a selective ligand that labels sigma-type receptors for psychotomimetic opiates and antipsychotic drugs. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8784–8788. doi: 10.1073/pnas.83.22.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts J., Balster R. L. The discriminative stimulus effects of N-methyl-D-aspartate antagonists in phencyclidine-trained rats. Neuropharmacology. 1988 Dec;27(12):1249–1256. doi: 10.1016/0028-3908(88)90027-5. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. H., Knight A. R., Woodruff G. N. [3H]MK-801 labels a site on the N-methyl-D-aspartate receptor channel complex in rat brain membranes. J Neurochem. 1988 Jan;50(1):274–281. doi: 10.1111/j.1471-4159.1988.tb13260.x. [DOI] [PubMed] [Google Scholar]


