Abstract
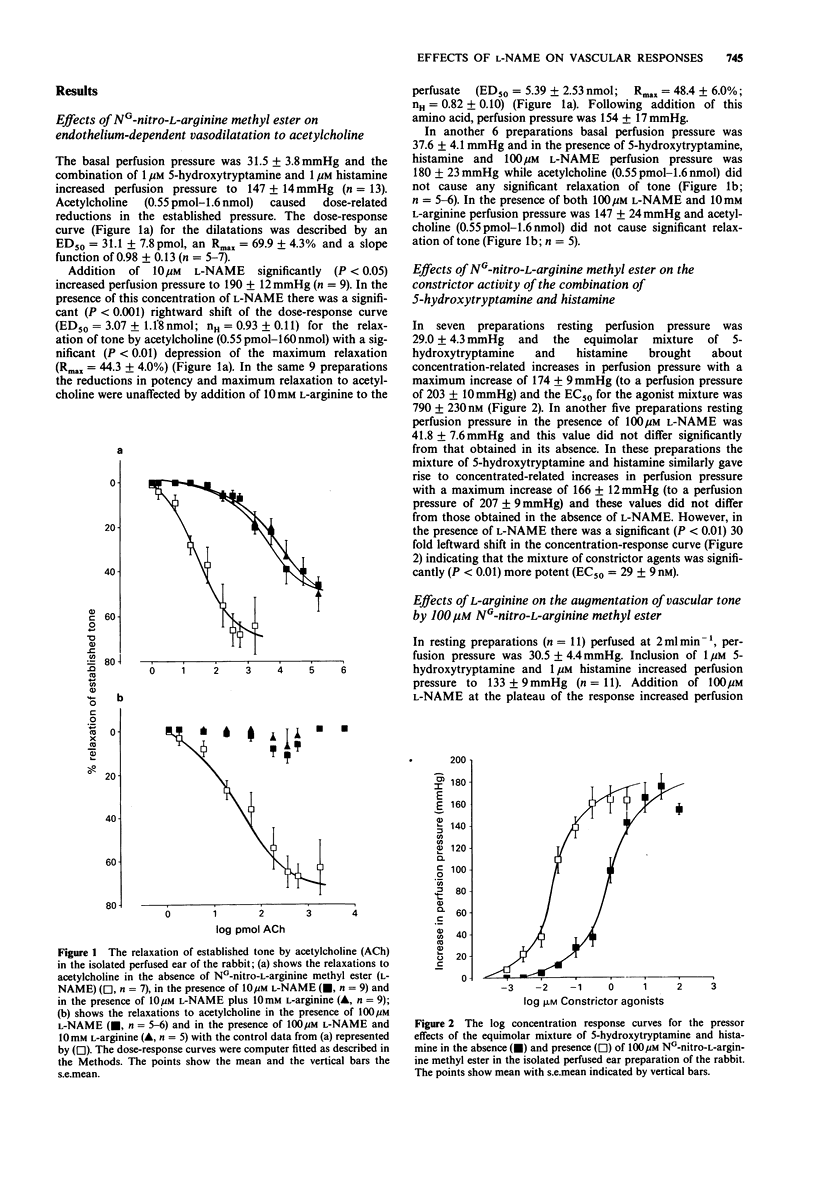
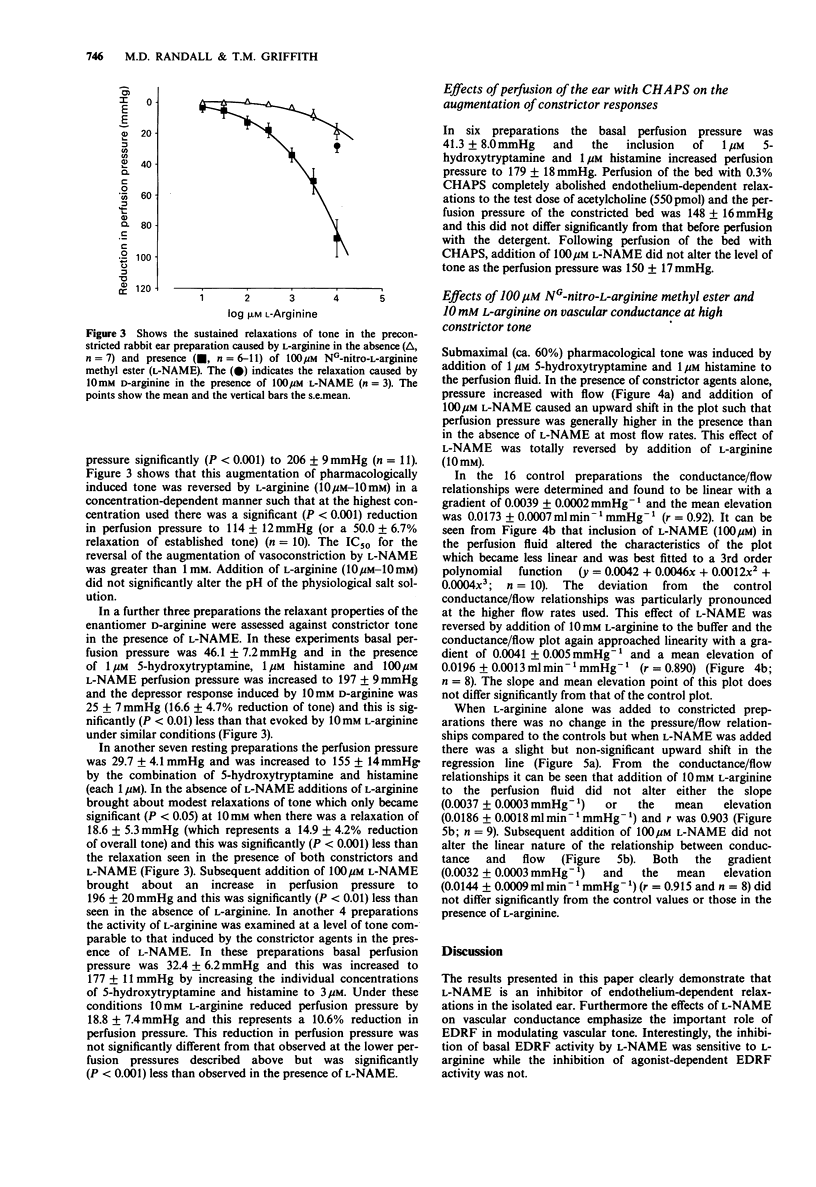
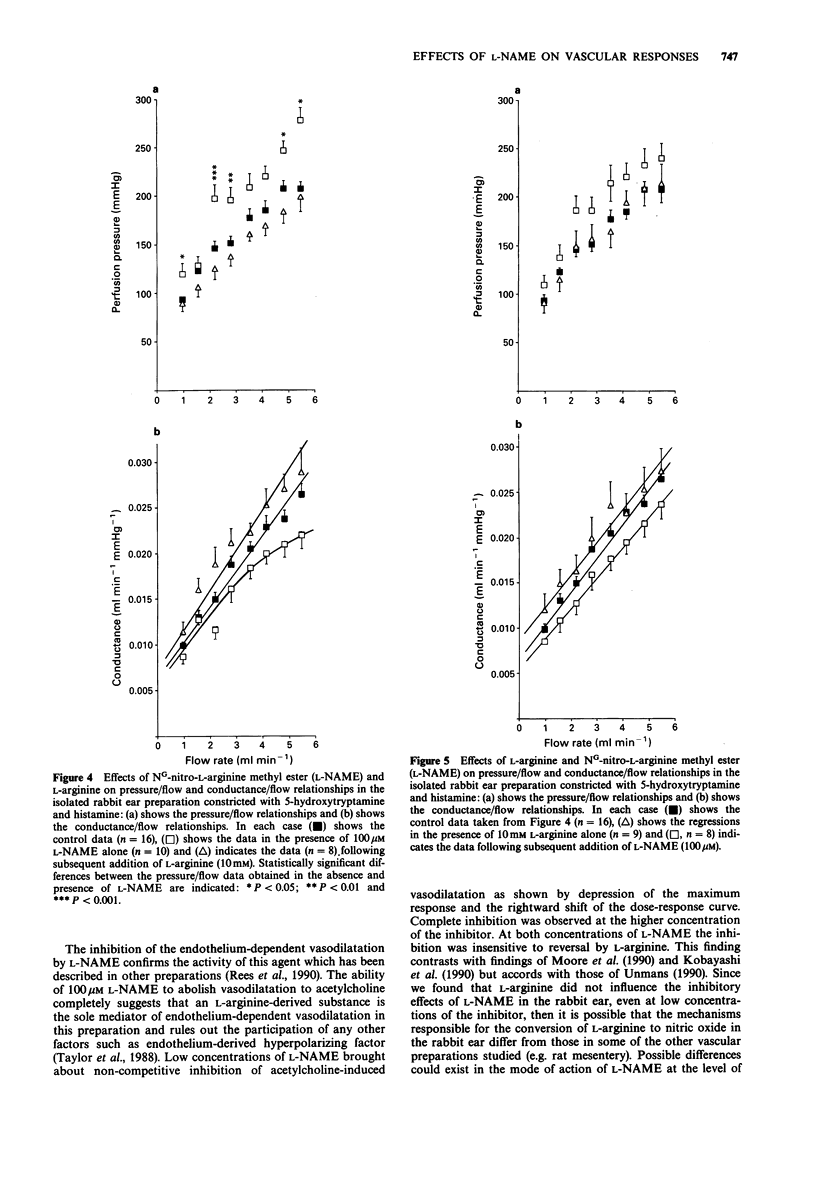
1. An isolated, buffer-perfused rabbit ear preparation was used to investigate the influence of NG-nitro-L-arginine methyl ester (L-NAME) on endothelium-dependent vasodiltation and modulation of vasoconstrictor responses and vascular conductance. 2. Acetylcholine (0.55 pmol-1.6 nmol) caused dose-related vasodilatation of preparations constricted by the combination of 5-hydroxytryptamine and histamine (both 1 microM), with an ED50 = 31.1 +/- 7.8 pmol and a maximum dilatation of 69.9 +/- 4.3%. In the presence of 10 microM L-NAME the dose-response for vasodilator effects was shifted significantly (P less than 0.001) to the right (ED50 = 3.07 +/- 1.18 nmol) and there was a significant (P less than 0.01) depression of the maximum response (Rmax = 44.3 +/- 4.0%). The higher concentration of 100 microM L-NAME completely abolished vasodilatation to acetylcholine. L-Arginine (10 mM) did not reverse the inhibitory actions of L-NAME at either concentration. 3. L-NAME 100 microM, augmented vascular tone induced by 1 microM 5-hydroxytryptamine and 1 microM histamine, thus altering the characteristics of both pressure/flow and conductance/flow relationships such that conductance was reduced at all flow rates. The augmentation of constrictor tone was reversed in a concentration-dependent manner by L-arginine (10 microM-10 mM) and the effect of L-NAME on the conductance/flow relationships was similarly reversed by 10 mM L-arginine. The augmentation of tone was endothelium-dependent as it did not occur following functional destruction of the endothelium by perfusion of the vascular bed with the detergent CHAPS (0.3%) for 150s.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceves J., Mariscal S., Morrison K. E., Young J. M. The binding of doxepin to histamine H1-receptors in guinea-pig and rat brain. Br J Pharmacol. 1985 Feb;84(2):417–424. doi: 10.1111/j.1476-5381.1985.tb12925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1990 Nov;101(3):625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. EDRF coordinates the behaviour of vascular resistance vessels. Nature. 1987 Oct 1;329(6138):442–445. doi: 10.1038/329442a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. Endothelium-derived relaxing factor (EDRF) and resistance vessels in an intact vascular bed: a microangiographic study of the rabbit isolated ear. Br J Pharmacol. 1988 Mar;93(3):654–662. doi: 10.1111/j.1476-5381.1988.tb10323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Henderson A. H. The role of EDRF in flow distribution: a microangiographic study of the rabbit isolated ear. Microvasc Res. 1989 Mar;37(2):162–177. doi: 10.1016/0026-2862(89)90035-6. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Henderson A. H. Unstimulated release of endothelium derived relaxing factor is independent of mitochondrial ATP generation. Cardiovasc Res. 1987 Aug;21(8):565–568. doi: 10.1093/cvr/21.8.565. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Henderson A. H., Edwards D. H., Lewis M. J. Isolated perfused rabbit coronary artery and aortic strip preparations: the role of endothelium-derived relaxant factor. J Physiol. 1984 Jun;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M., Feelisch M., Spahr R., Piper H. M., Noack E., Schrader J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- Kosuga K., Yui Y., Hattori R., Eizawa H., Hiki K., Kawai C. Stabilizing factor(s) of nitric oxide (NO) synthetase. Biochem Biophys Res Commun. 1990 Oct 30;172(2):705–708. doi: 10.1016/0006-291x(90)90731-2. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Characterization of the L-arginine:nitric oxide pathway in human platelets. Br J Pharmacol. 1990 Oct;101(2):325–328. doi: 10.1111/j.1476-5381.1990.tb12709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. D., Edwards D. H., Griffith T. M. Activities of endothelin-1 in the vascular network of the rabbit ear: a microangiographic study. Br J Pharmacol. 1990 Dec;101(4):781–788. doi: 10.1111/j.1476-5381.1990.tb14157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. D., Hiley C. R. Detergent and methylene blue affect endothelium-dependent vasorelaxation and pressure/flow relations in rat blood perfused mesenteric arterial bed. Br J Pharmacol. 1988 Dec;95(4):1081–1088. doi: 10.1111/j.1476-5381.1988.tb11742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. D., Hiley C. R. Effect of phenobarbitone pretreatment upon endothelium-dependent relaxation to acetylcholine in rat superior mesenteric arterial bed. Br J Pharmacol. 1988 Jul;94(3):977–983. doi: 10.1111/j.1476-5381.1988.tb11612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. G., Southerton J. S., Weston A. H., Baker J. R. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol. 1988 Jul;94(3):853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Hecker M., Ramwell P. W. Vascular activity of polycations and basic amino acids: L-arginine does not specifically elicit endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Jan 16;158(1):177–180. doi: 10.1016/s0006-291x(89)80194-9. [DOI] [PubMed] [Google Scholar]
- Tucker J. F., Brave S. R., Charalambous L., Hobbs A. J., Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990 Aug;100(4):663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


