Abstract
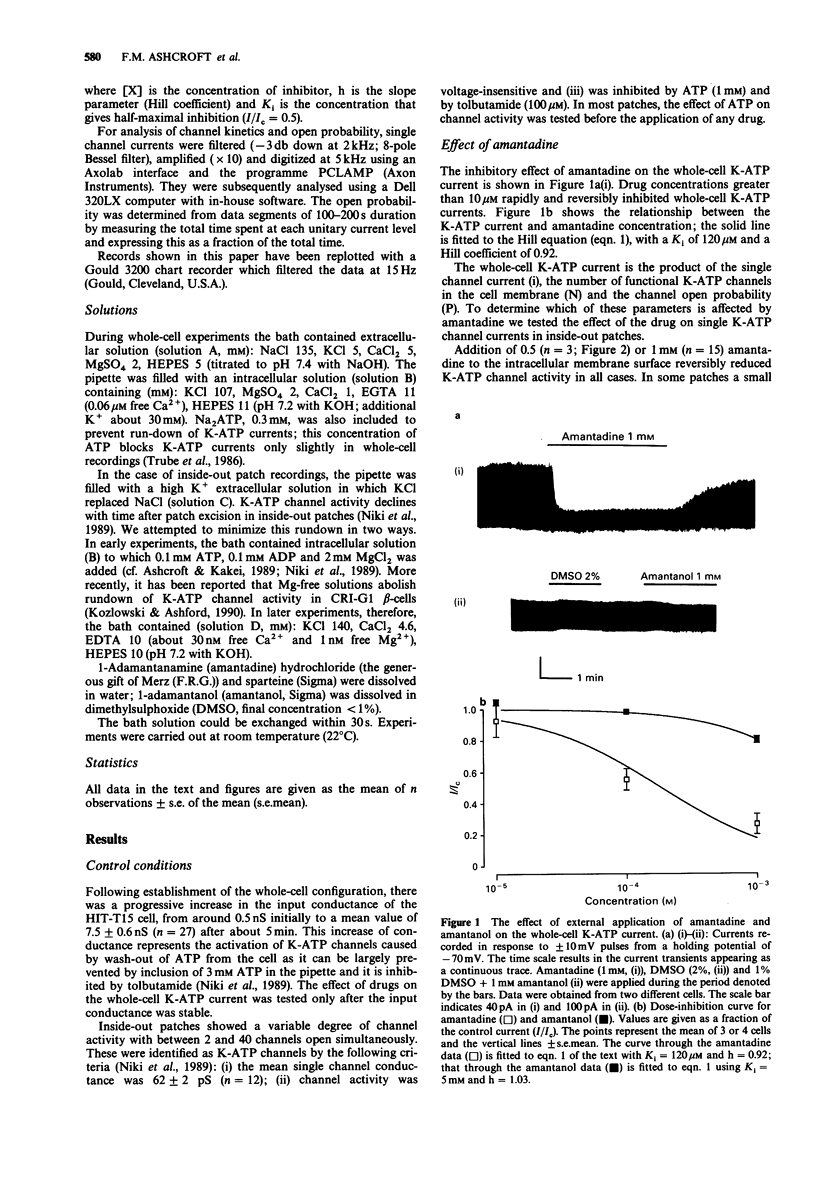
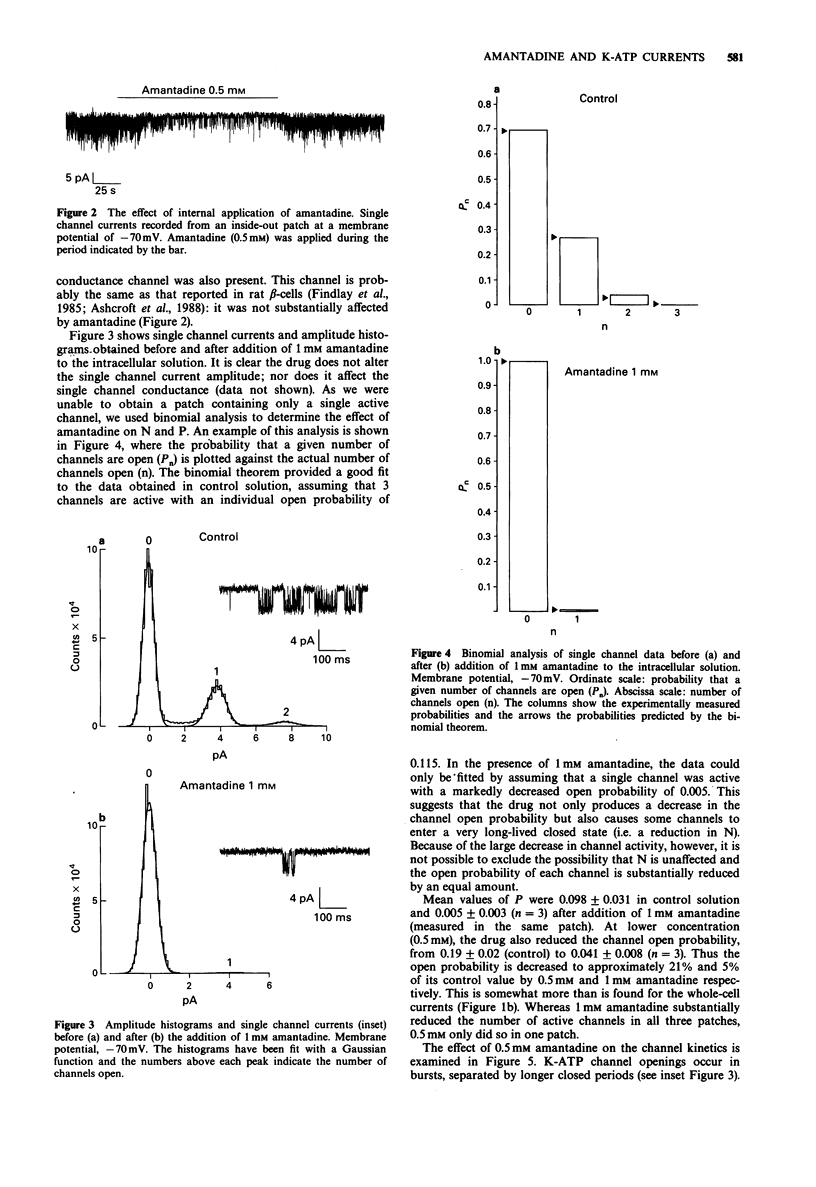
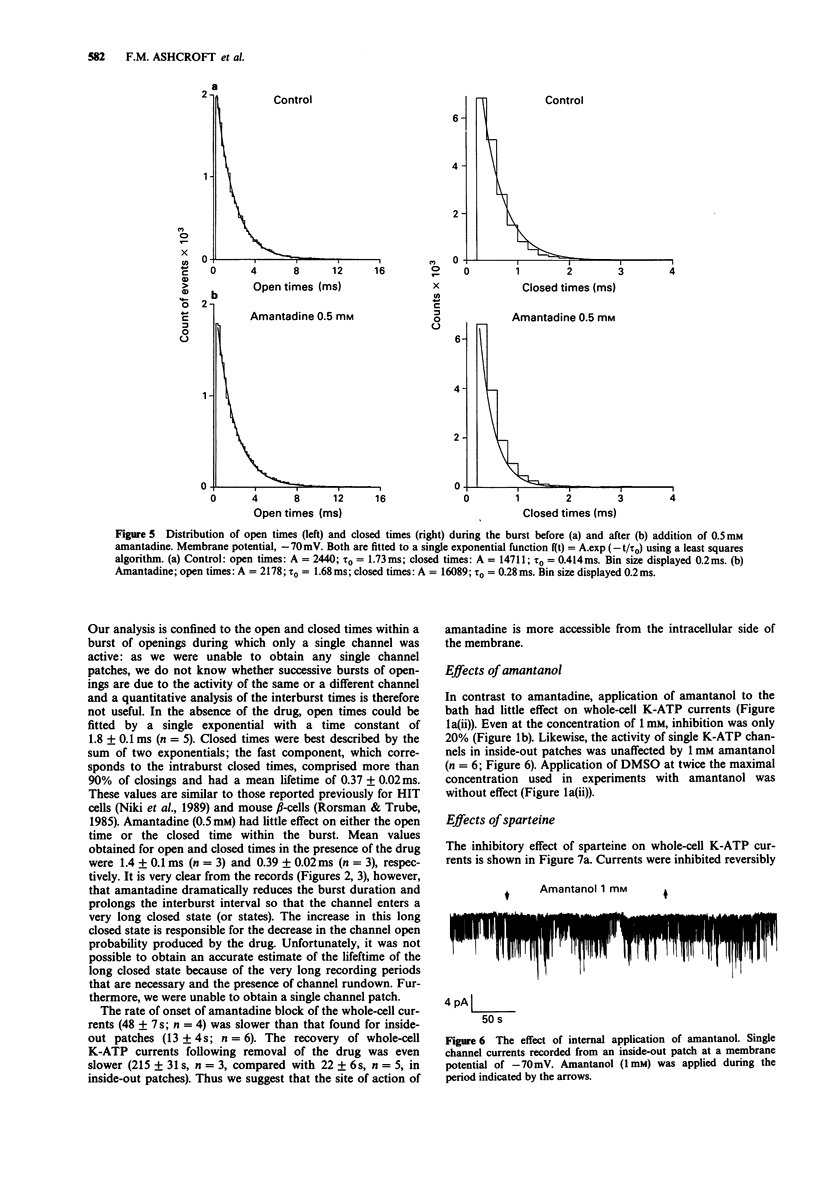
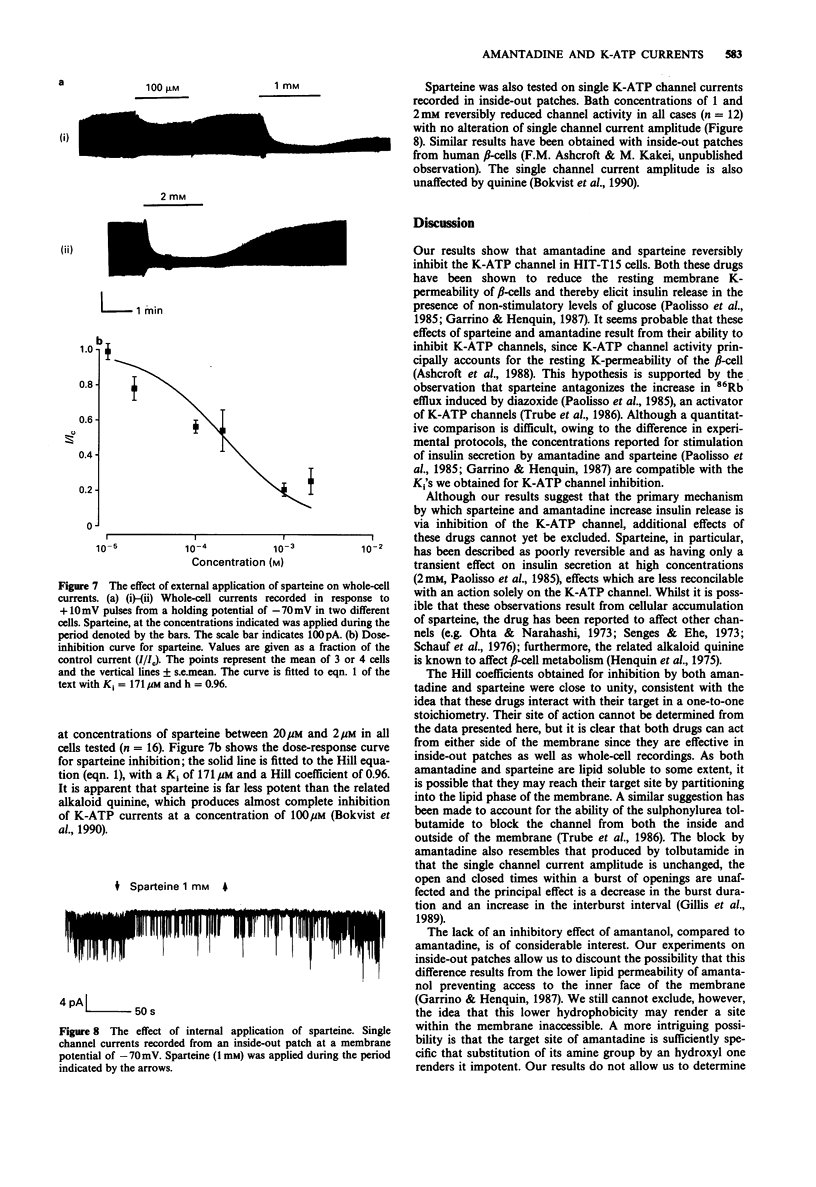
1. The effects of pharmacological agents that potentiate insulin release were studied on ATP-regulated K-currents (K-ATP currents) in the insulin-secreting beta-cell line HIT-T15 by use of patch-clamp methods. 2. The tricyclic drug, 1-adamantanamine (amantadine), reversibly inhibited both whole-cell currents (with a Ki of 120 microM) and single channel currents in inside-out patches. This effect was principally due to an increase in a long closed state which reduced the channel open probability. The related compound, 1-adamantanol, in which the amino group is substituted by a hydroxyl one, did not inhibit K-ATP currents substantially. 3. The alkaloid, sparteine, reversibly inhibited both whole-cell K-ATP currents (Ki = 171 microM) and single channel currents in inside-out patches. 4. The results suggest that sparteine and amantadine can block the K-ATP channel from either side of the membrane and support the idea that at least part of the stimulatory effect of these agents on insulin secretion results from inhibition of this channel.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amoroso S., Schmid-Antomarchi H., Fosset M., Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990 Feb 16;247(4944):852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Properties of single potassium channels modulated by glucose in rat pancreatic beta-cells. J Physiol. 1988 Jun;400:501–527. doi: 10.1113/jphysiol.1988.sp017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Kakei M. ATP-sensitive K+ channels in rat pancreatic beta-cells: modulation by ATP and Mg2+ ions. J Physiol. 1989 Sep;416:349–367. doi: 10.1113/jphysiol.1989.sp017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Hammonds P., Harrison D. E. Insulin secretory responses of a clonal cell line of simian virus 40-transformed B cells. Diabetologia. 1986 Oct;29(10):727–733. doi: 10.1007/BF00870283. [DOI] [PubMed] [Google Scholar]
- Bokvist K., Rorsman P., Smith P. A. Effects of external tetraethylammonium ions and quinine on delayed rectifying K+ channels in mouse pancreatic beta-cells. J Physiol. 1990 Apr;423:311–325. doi: 10.1113/jphysiol.1990.sp018024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Petersen O. H. ATP-sensitive inward rectifier and voltage- and calcium-activated K+ channels in cultured pancreatic islet cells. J Membr Biol. 1985;88(2):165–172. doi: 10.1007/BF01868430. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Henquin J. C. Adamantane derivatives: a new class of insulin secretagogues. Br J Pharmacol. 1987 Mar;90(3):583–591. doi: 10.1111/j.1476-5381.1987.tb11209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Horemans B., Nenquin M., Verniers J., Lambert A. E. Quinine-induced modifications of insulin release and glucose metabolism by isolated pancreatic islets. FEBS Lett. 1975 Oct 1;57(3):280–284. doi: 10.1016/0014-5793(75)80317-6. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Misler S., Falke L. C., Gillis K., McDaniel M. L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki I., Kelly R. P., Ashcroft S. J., Ashcroft F. M. ATP-sensitive K-channels in HIT T15 beta-cells studied by patch-clamp methods, 86Rb efflux and glibenclamide binding. Pflugers Arch. 1989 Oct;415(1):47–55. doi: 10.1007/BF00373140. [DOI] [PubMed] [Google Scholar]
- Ota M., Narahashi T. Sparteine interaction with nerve membrane potassium conductance. J Pharmacol Exp Ther. 1973 Oct;187(1):47–55. [PubMed] [Google Scholar]
- Paolisso G., Nenquin M., Schmeer W., Mathot F., Meissner H. P., Henquin J. C. Sparteine increases insulin release by decreasing the K+ permeability of the B-cell membrane. Biochem Pharmacol. 1985 Jul 1;34(13):2355–2361. doi: 10.1016/0006-2952(85)90794-4. [DOI] [PubMed] [Google Scholar]
- Parkes D. Amantadine. Adv Drug Res. 1974;8:11–81. [PubMed] [Google Scholar]
- Rorsman P., Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985 Dec;405(4):305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Colton C. A., Colton J. S., Davis F. A. Aminopyridines and sparteine as inhibitors of membrane potassium conductance: effects on Myxicola giant axons and the lobster neuromuscular junction. J Pharmacol Exp Ther. 1976 May;197(2):414–425. [PubMed] [Google Scholar]
- Senges J., Ehe L. The effects of sparteine on membrane potential and contraction of mammalian heart muscle. Naunyn Schmiedebergs Arch Pharmacol. 1973;280(3):253–264. doi: 10.1007/BF00501350. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]


