Abstract
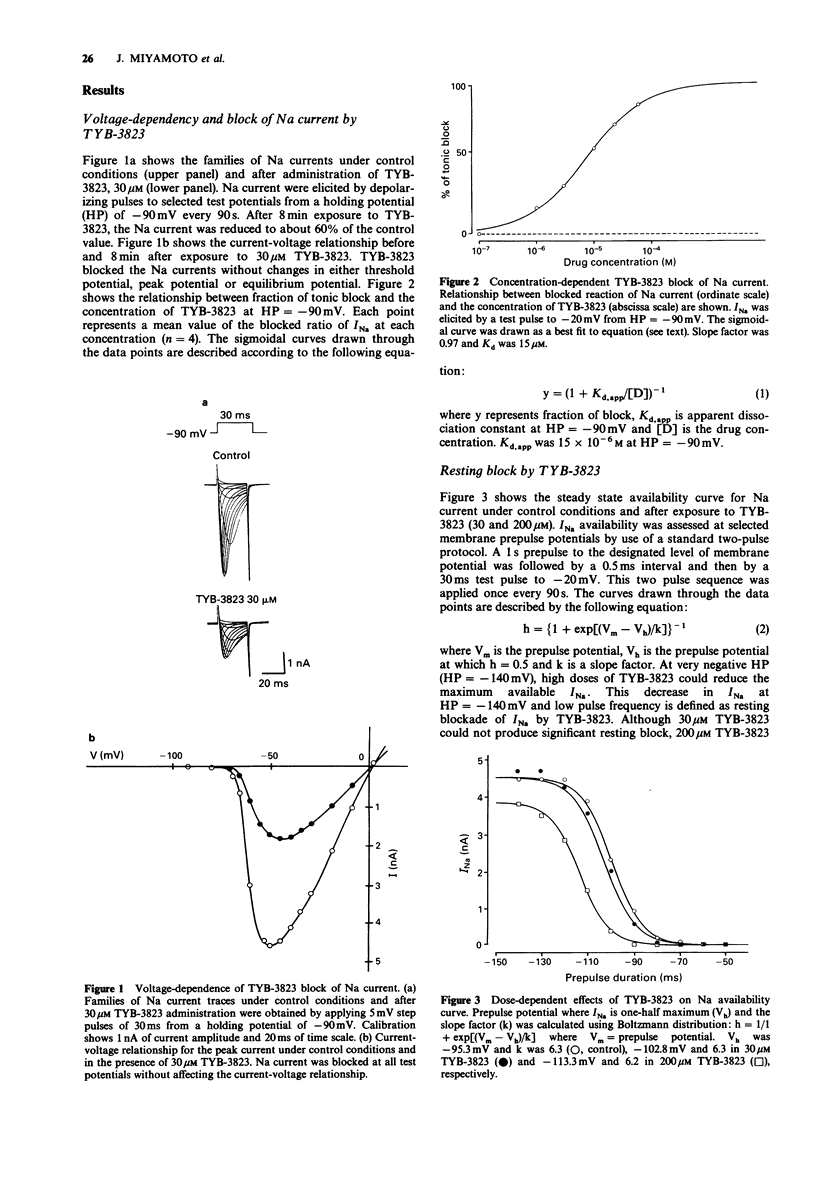
1. Sodium current (INa) blockade by TYB-3823, a newly synthesized antiarrhythmic agent, was investigated in isolated single ventricular myocytes by use of the whole cell patch-clamp technique. 2. TYB-3823 blocked INa under steady-state conditions (Kd,rest = 500 microM, Kd,i = 4.9 microM), findings consistent with a shift in the steady state INa availability curve to more negative potentials. 3. TYB-3823 produced use-dependent block at 2 Hz in conjunction with increase in pulse duration (5-300 ms), that was markedly enhanced at less negative holding potentials. 4. The time course of the onset of block was accelerated and the degree of use-dependent block was decreased at more negative holding potential. The time course of the onset of block was accentuated with enhancing block at more positive holding potentials. 5. The time course of recovery from use-dependent block was accelerated at more negative holding potentials but was accentuated at more positive holding potentials. 6. These results suggest that both tonic block and use-dependent block of sodium channels in cardiac tissue might result from an interaction of TYB-3832 with sodium channels mainly in the inactivated channel states and the kinetics of the interaction between drug and receptor may be modulated by the inactivation gate.
Full text
PDF
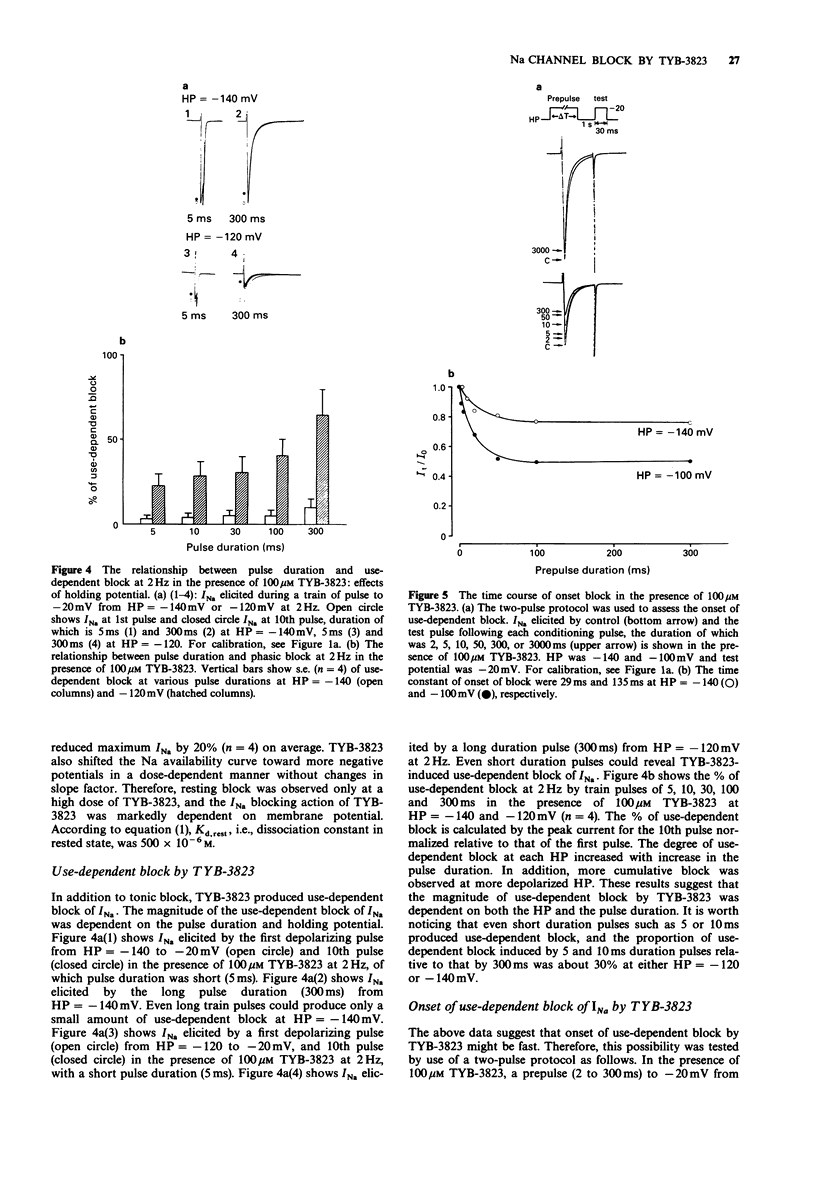
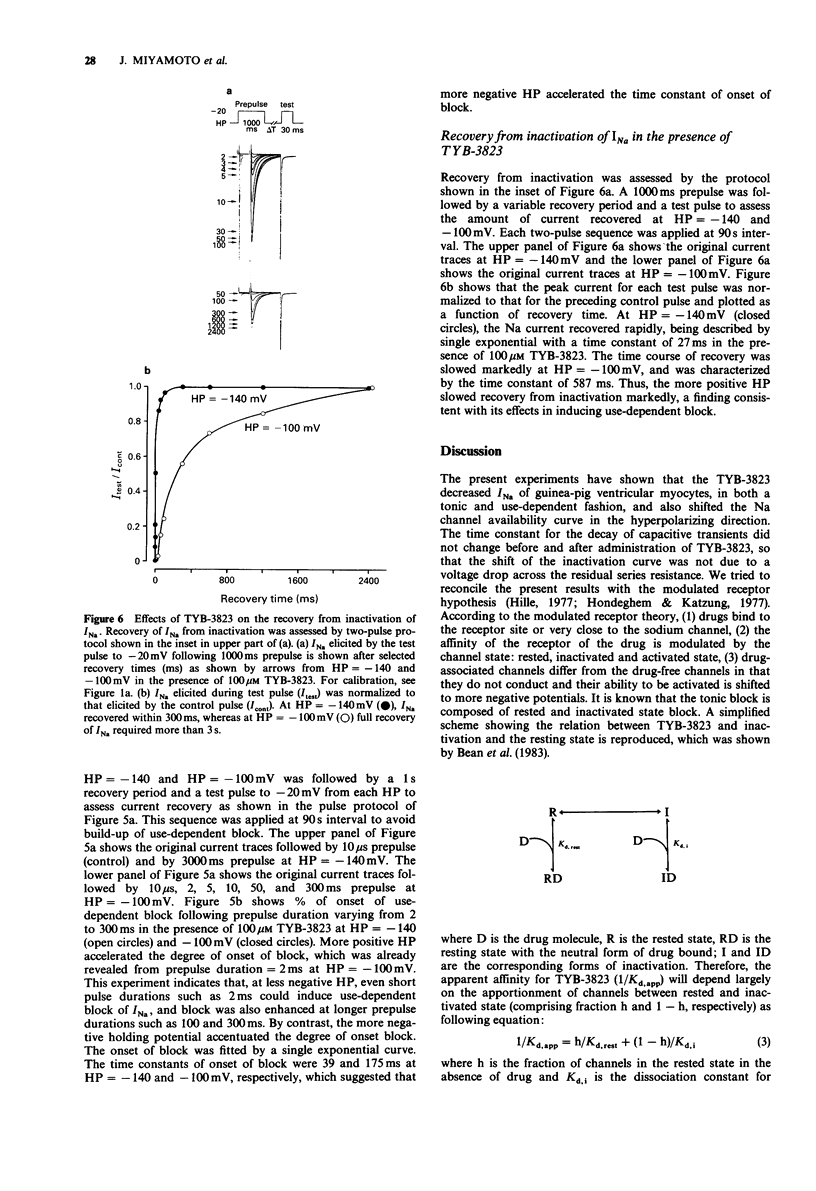


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., Bean B. P., Tsien R. W. Maximal upstroke velocity as an index of available sodium conductance. Comparison of maximal upstroke velocity and voltage clamp measurements of sodium current in rabbit Purkinje fibers. Circ Res. 1984 Jun;54(6):636–651. doi: 10.1161/01.res.54.6.636. [DOI] [PubMed] [Google Scholar]
- Colatsky J. J., Tsien R. W. Sodium channels in rabbit cardiac Purkinje fibres. Nature. 1979 Mar 15;278(5701):265–268. doi: 10.1038/278265a0. [DOI] [PubMed] [Google Scholar]
- Ehara T., Matsuoka S., Noma A. Measurement of reversal potential of Na+-Ca2+ exchange current in single guinea-pig ventricular cells. J Physiol. 1989 Mar;410:227–249. doi: 10.1113/jphysiol.1989.sp017530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatome I., Matsuoka S., Miyamoto J., Sawaguchi M., Omodani H., Osaki S., Kotake H., Mashiba H., Sato R. Blocking effect of 1389-S on the sodium current in isolated guinea-pig ventricular myocytes. Eur J Pharmacol. 1990 Apr 25;179(3):447–451. doi: 10.1016/0014-2999(90)90187-b. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Kodama I., Morimoto M., Toyama J., Shibata S. Electrophysiologic effects of TYB-3823, a new antiarrhythmic agent, on isolated guinea pig ventricular muscles. J Cardiovasc Pharmacol. 1989 Apr;13(4):616–623. [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer C. F., Grant A. O., Strauss H. C. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J. 1984 Jul;46(1):15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel activation in the squid giant axon. Steady state properties. J Gen Physiol. 1985 Jan;85(1):65–82. doi: 10.1085/jgp.85.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy J., Yeh J. Z. Batrachotoxin uncouples gating charge immobilization from fast Na inactivation in squid giant axons. Biophys J. 1988 Oct;54(4):719–730. doi: 10.1016/S0006-3495(88)83007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


