Abstract
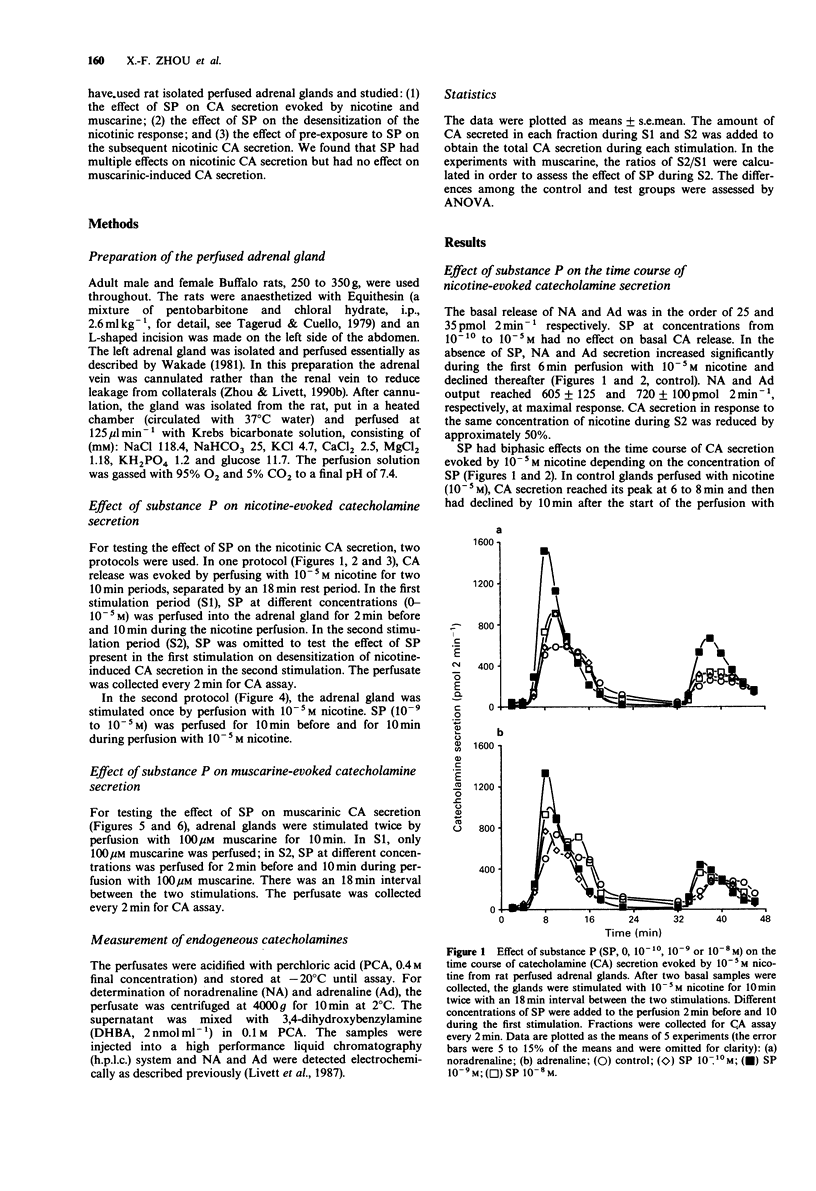
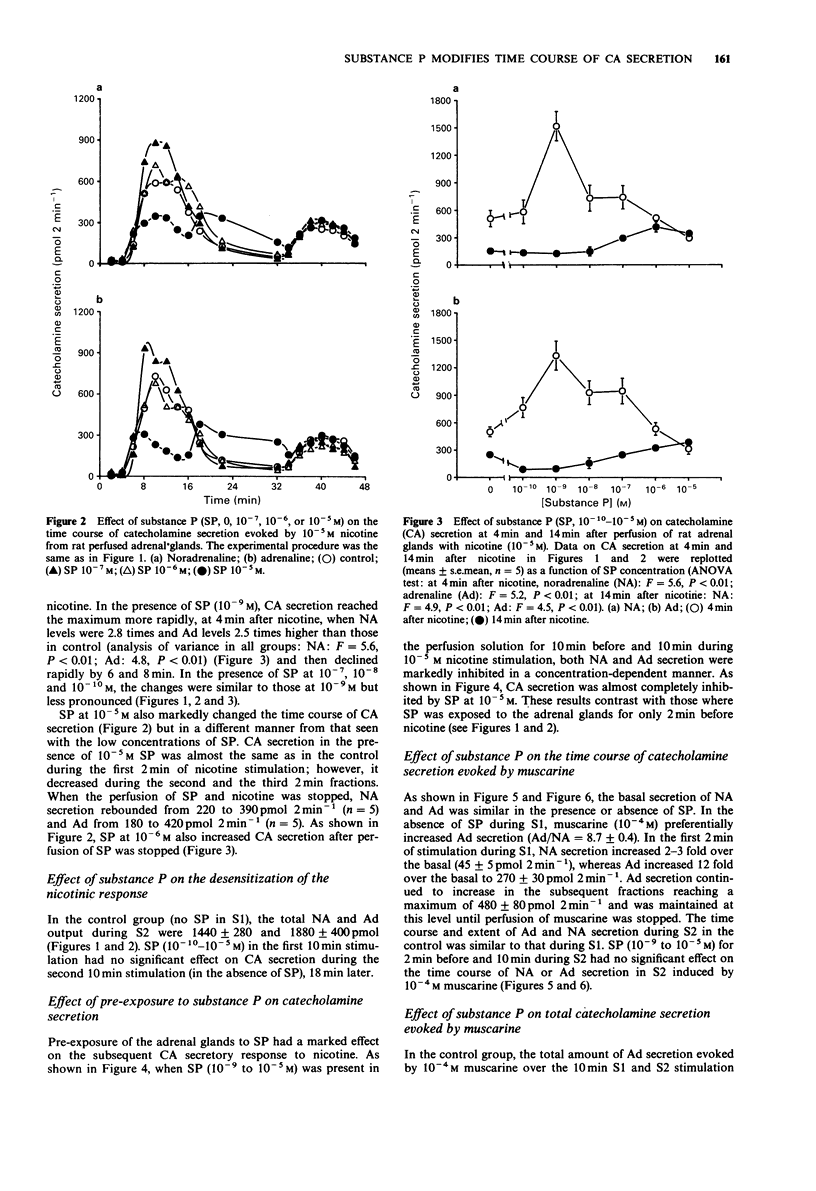
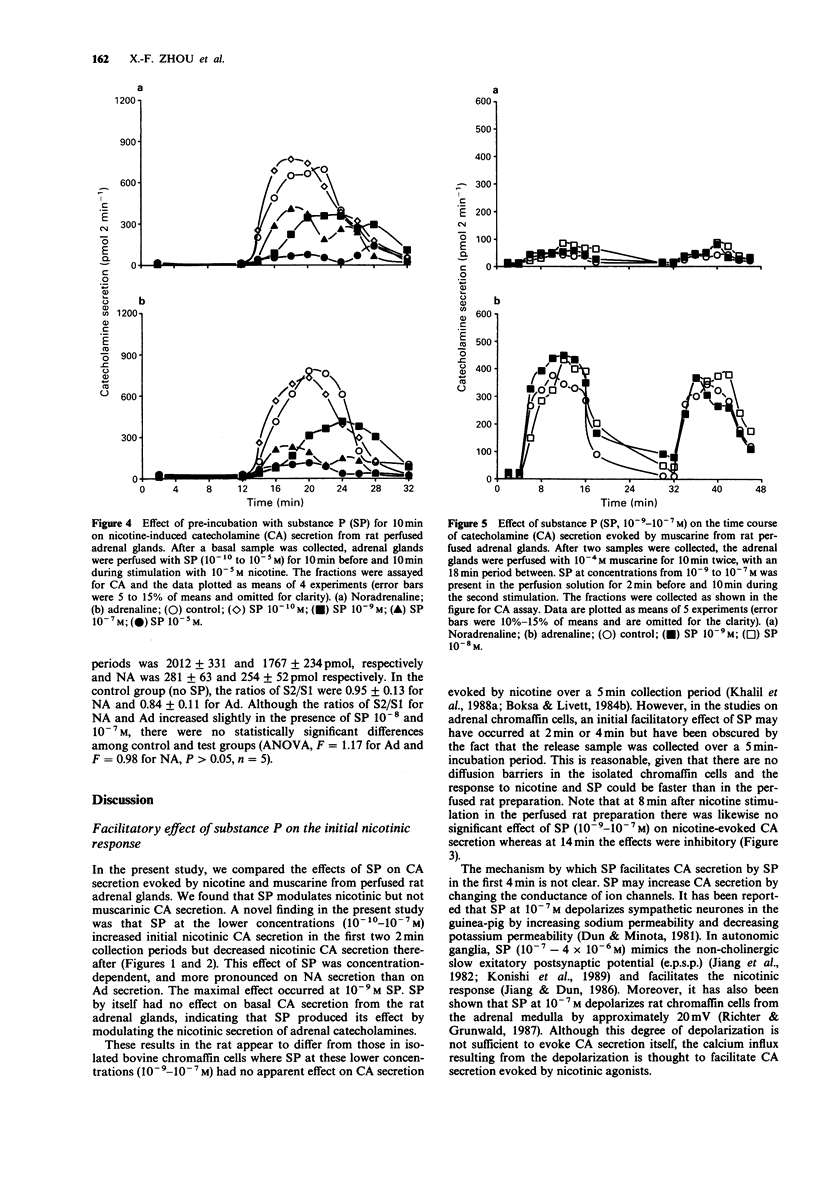
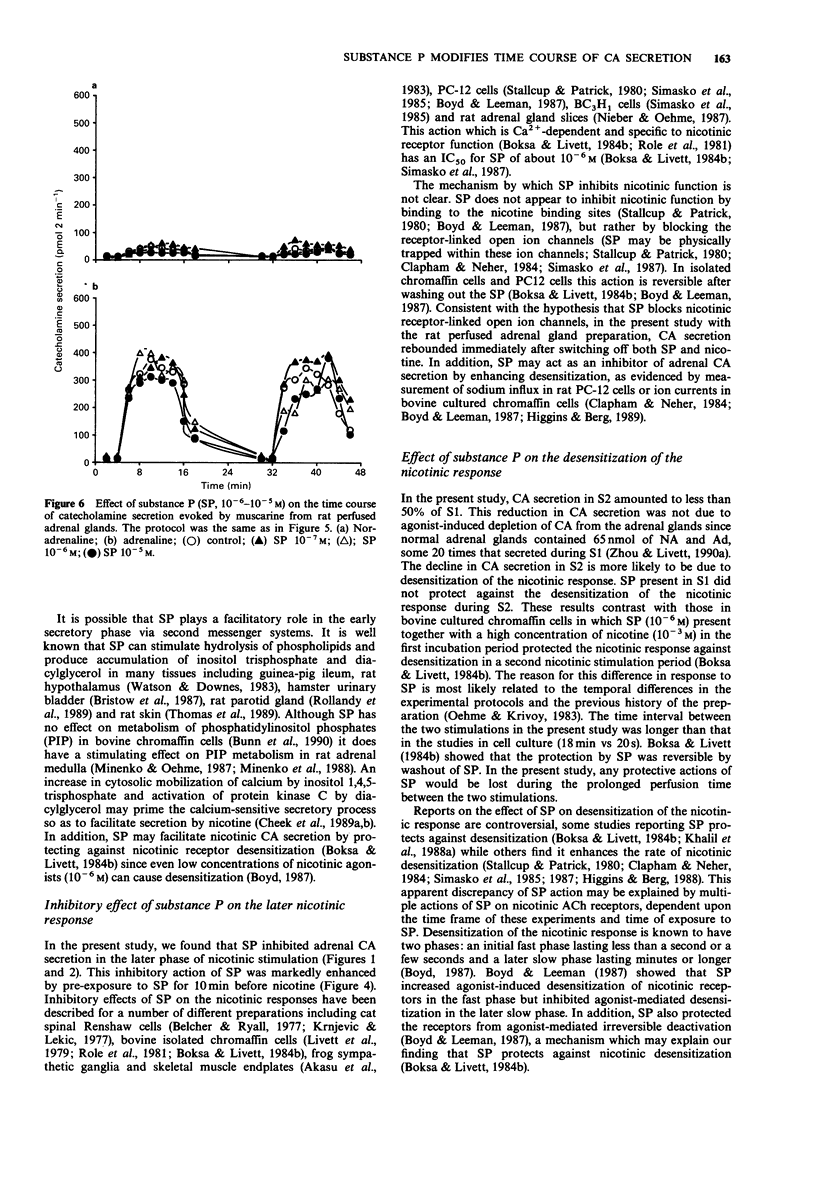
1. Substance P (SP) and acetylcholine (ACh) are contained within the splanchnic nerve terminals in the adrenal gland and can be released in response to stress. In the rat, the release of aCh brings about secretion of catecholamines (CA) by acting on nicotinic and muscarinic receptors on the adrenal chromaffin cells. 2. In the present study, we have used a rat isolated adrenal gland preparation to investigate the effects of SP, perfused at different concentrations, on CA secretion evoked by 10(-5) M nicotine and 10(-4) M muscarine. 3. In the first 10 min stimulation period (S1), in the absence of SP, nicotine (10(-5) M) evoked substantial and equal secretion of noradrenaline (NA) and adrenaline (Ad). In a second 10 min stimulation period (S2), carried out 18 min after S1, the nicotinic response was desensitized. In contrast, the muscarinic response, which preferentially evoked Ad secretion in S1 (Ad/NA: 8.7/1), was well maintained in S2. 4. SP present in S1 had no effect on desensitization of the subsequent nicotinic response in S2. 5. At low concentrations (10(-7)-10(-10) M), SP changed the time course of nicotine-induced CA secretion during S1 by enhancing CA secretion in the first 4 min and inhibiting CA secretion thereafter. The maximal effect occurred at 10(-9) M SP. 6. At a higher concentration (10(-5) M), SP inhibited total nicotinic CA secretion throughout S1 and produced a biphasic secretion of CA (depressed in the presence of SP and enhanced after wash out of SP).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Kojima M., Koketsu K. Substance P modulates the sensitivity of the nicotinic receptor in amphibian cholinergic transmission. Br J Pharmacol. 1983 Sep;80(1):123–131. doi: 10.1111/j.1476-5381.1983.tb11057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W. Substance P and Renshaw cells: a new concept of inhibitory synaptic interactions. J Physiol. 1977 Oct;272(1):105–119. doi: 10.1113/jphysiol.1977.sp012036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P., Livett B. G. Desensitization to nicotinic cholinergic agonists and K+, agents that stimulate catecholamine secretion, in isolated adrenal chromaffin cells. J Neurochem. 1984 Mar;42(3):607–617. doi: 10.1111/j.1471-4159.1984.tb02726.x. [DOI] [PubMed] [Google Scholar]
- Boksa P., Livett B. G. Substance P protects against desensitization of the nicotinic response in isolated adrenal chromaffin cells. J Neurochem. 1984 Mar;42(3):618–627. doi: 10.1111/j.1471-4159.1984.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., Leeman S. E. Multiple actions of substance P that regulate the functional properties of acetylcholine receptors of clonal rat PC12 cells. J Physiol. 1987 Aug;389:69–97. doi: 10.1113/jphysiol.1987.sp016647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N. D. Two distinct kinetic phases of desensitization of acetylcholine receptors of clonal rat PC12 cells. J Physiol. 1987 Aug;389:45–67. doi: 10.1113/jphysiol.1987.sp016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow D. R., Curtis N. R., Suman-Chauhan N., Watling K. J., Williams B. J. Effects of tachykinins on inositol phospholipid hydrolysis in slices of hamster urinary bladder. Br J Pharmacol. 1987 Jan;90(1):211–217. doi: 10.1111/j.1476-5381.1987.tb16842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn S. J., Marley P. D., Livett B. G. Receptor stimulated formation of inositol phosphates in cultures of bovine adrenal medullary cells: the effects of bradykinin, bombesin and neurotensin. Neuropeptides. 1990 Apr;15(4):187–194. doi: 10.1016/0143-4179(90)90012-n. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells. Distinct nicotinic and muscarinic patterns. FEBS Lett. 1989 Apr 24;247(2):429–434. doi: 10.1016/0014-5793(89)81385-7. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Thastrup O. Internal Ca2+ mobilization and secretion in bovine adrenal chromaffin cells. Cell Calcium. 1989 May-Jun;10(4):213–221. doi: 10.1016/0143-4160(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley J. A., Ellis P., Henderson C. G., Ungar A., West C. P. Muscarinic and nicotinic mechanisms in the responses of the adrenal medulla of the dog and cat to reflex stimuli and to cholinomimetic drugs. Br J Pharmacol. 1986 Dec;89(4):831–835. doi: 10.1111/j.1476-5381.1986.tb11188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Effects of substance P on neurones of the inferior mesenteric ganglia of the guinea-pig. J Physiol. 1981 Dec;321:259–271. doi: 10.1113/jphysiol.1981.sp013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Minz B., Tsudzimura H. The mechanism of the nervous discharge of adrenaline. J Physiol. 1934 Jun 9;81(3):286–304. doi: 10.1113/jphysiol.1934.sp003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish O. E., Kao L. S., Raffaniello R., Wakade A. R., Schneider A. S. Calcium dependence of muscarinic receptor-mediated catecholamine secretion from the perfused rat adrenal medulla. J Neurochem. 1987 Jun;48(6):1730–1735. doi: 10.1111/j.1471-4159.1987.tb05730.x. [DOI] [PubMed] [Google Scholar]
- Higgins L. S., Berg D. K. A desensitized form of neuronal acetylcholine receptor detected by 3H-nicotine binding on bovine adrenal chromaffin cells. J Neurosci. 1988 Apr;8(4):1436–1446. doi: 10.1523/JNEUROSCI.08-04-01436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. G., Dun N. J. Facilitation of nicotinic response in the guinea pig prevertebral neurons by substance P. Brain Res. 1986 Jan 15;363(1):196–198. doi: 10.1016/0006-8993(86)90679-7. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Dun N. J., Karczmar A. G. Substance P: a putative sensory transmitter in mammalian autonomic ganglia. Science. 1982 Aug 20;217(4561):739–741. doi: 10.1126/science.6179162. [DOI] [PubMed] [Google Scholar]
- Khalil Z., Marley P. D., Livett B. G. Effect of substance P on nicotine-induced desensitization of cultured bovine adrenal chromaffin cells: possible receptor subtypes. Brain Res. 1988 Sep 6;459(2):282–288. doi: 10.1016/0006-8993(88)90644-0. [DOI] [PubMed] [Google Scholar]
- Khalil Z., Marley P. D., Livett B. G. Mammalian tachykinins modulate the nicotinic secretory response of cultured bovine adrenal chromaffin cells. Brain Res. 1988 Sep 6;459(2):289–297. doi: 10.1016/0006-8993(88)90645-2. [DOI] [PubMed] [Google Scholar]
- Kong J. Y., Thureson-Klein A., Klein R. L. Differential distribution of neuropeptides and serotonin in pig adrenal glands. Neuroscience. 1989;28(3):765–775. doi: 10.1016/0306-4522(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Konishi S., Song S. Y., Ogawa T., Kanazawa I. Tachykinins produce fast and slow depolarizations in sympathetic neurons of rat coeliac-superior mesenteric ganglia. Brain Res. 1989 Jun 19;490(1):162–165. doi: 10.1016/0006-8993(89)90444-7. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Lekić D. Substance P selectively blocks excitation of Renshaw cell by acetylcholine. Can J Physiol Pharmacol. 1977 Aug;55(4):958–961. doi: 10.1139/y77-129. [DOI] [PubMed] [Google Scholar]
- Kuramoto H., Kondo H., Fujita T. Calcitonin gene-related peptide (CGRP)-like immunoreactivity in scattered chromaffin cells and nerve fibers in the adrenal gland of rats. Cell Tissue Res. 1987 Feb;247(2):309–315. doi: 10.1007/BF00218312. [DOI] [PubMed] [Google Scholar]
- Linnoila R. I., Diaugustine R. P., Hervonen A., Miller R. J. Distribution of [Met5]- and [Leu5]-enkephalin-, vasoactive intestinal polypeptide- and substance P-like immunoreactivities in human adrenal glands. Neuroscience. 1980;5(12):2247–2259. doi: 10.1016/0306-4522(80)90141-4. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Kozousek V., Mizobe F., Dean D. M. Substance P inhibits nicotinic activation of chromaffin cells. Nature. 1979 Mar 15;278(5701):256–257. doi: 10.1038/278256a0. [DOI] [PubMed] [Google Scholar]
- Marley P., Livett B. G. Neuropeptides in the autonomic nervous system. CRC Crit Rev Clin Neurobiol. 1985;1(3):201–283. [PubMed] [Google Scholar]
- Minenko A., Gabrysiak B., Oehme P. Decreased SP-stimulated diesteratic hydrolysis of inositol phospholipids in adrenal medulla slices from spontaneously hypertensive rats. Biomed Biochim Acta. 1988;47(1):31–37. [PubMed] [Google Scholar]
- Minenko A., Oehme P. Substance P action on inositol phospholipids in rat adrenal medulla slices. Biomed Biochim Acta. 1987;46(6):461–467. [PubMed] [Google Scholar]
- Nieber K., Oehme P. Effect of substance P (SP) and the N-terminal SP-analogue SP (1-4) on the pre- and postsynaptic transmitter release in rat adrenal gland slices. Biomed Biochim Acta. 1987;46(1):103–109. [PubMed] [Google Scholar]
- Richter R., Grunwald C. Effect of substance P on the membrane potential of rat adrenal chromaffin cells. Biomed Biochim Acta. 1987;46(11):837–840. [PubMed] [Google Scholar]
- Role L. W., Leeman S. E., Perlman R. L. Somatostatin and substance P inhibit catecholamine secretion from isolated cells of guinea-pig adrenal medulla. Neuroscience. 1981;6(9):1813–1821. doi: 10.1016/0306-4522(81)90215-3. [DOI] [PubMed] [Google Scholar]
- Rollandy I., Dreux C., Imhoff V., Rossignol B. Importance of the presence of the N-terminal tripeptide of substance P for the stimulation of phosphatidylinositol metabolism in rat parotid gland: a possible activation of phospholipases C and D. Neuropeptides. 1989 Apr;13(3):175–185. doi: 10.1016/0143-4179(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Simasko S. M., Durkin J. A., Weiland G. A. Effects of substance P on nicotinic acetylcholine receptor function in PC12 cells. J Neurochem. 1987 Jul;49(1):253–260. doi: 10.1111/j.1471-4159.1987.tb03423.x. [DOI] [PubMed] [Google Scholar]
- Simasko S. M., Soares J. R., Weiland G. A. Structure-activity relationship for substance P inhibition of carbamylcholine-stimulated 22Na+ flux in neuronal (PC12) and non-neuronal (BC3H1) cell lines. J Pharmacol Exp Ther. 1985 Dec;235(3):601–605. [PubMed] [Google Scholar]
- Stallcup W. B., Patrick J. Substance P enhances cholinergic receptor desensitization in a clonal nerve cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):634–638. doi: 10.1073/pnas.77.1.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagerud S. E., Cuello A. C. Dopamine release from the rat substantia nigra in vitro. Effect of raphe lesions and veratridine stimulation. Neuroscience. 1979;4(12):2021–2029. doi: 10.1016/0306-4522(79)90073-3. [DOI] [PubMed] [Google Scholar]
- Thomas K. L., Andrews P. V., Khalil Z., Helme R. D. Substance P induced hydrolysis of inositol phospholipids in rat skin in an in vivo model of inflammation. Neuropeptides. 1989 Apr;13(3):191–196. doi: 10.1016/0143-4179(89)90091-7. [DOI] [PubMed] [Google Scholar]
- Vaupel R., Jarry H., Schlömer H. T., Wuttke W. Differential response of substance P-containing subtypes of adrenomedullary cells to different stressors. Endocrinology. 1988 Oct;123(4):2140–2145. doi: 10.1210/endo-123-4-2140. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., Downes C. P. Substance P induced hydrolysis of inositol phospholipids in guinea-pig ileum and rat hypothalamus. Eur J Pharmacol. 1983 Sep 30;93(3-4):245–253. doi: 10.1016/0014-2999(83)90144-9. [DOI] [PubMed] [Google Scholar]
- Zhou X. F., Livett B. G. Substance P increases catecholamine secretion from perfused rat adrenal glands evoked by prolonged field stimulation. J Physiol. 1990 Jun;425:321–334. doi: 10.1113/jphysiol.1990.sp018105. [DOI] [PMC free article] [PubMed] [Google Scholar]


