Abstract
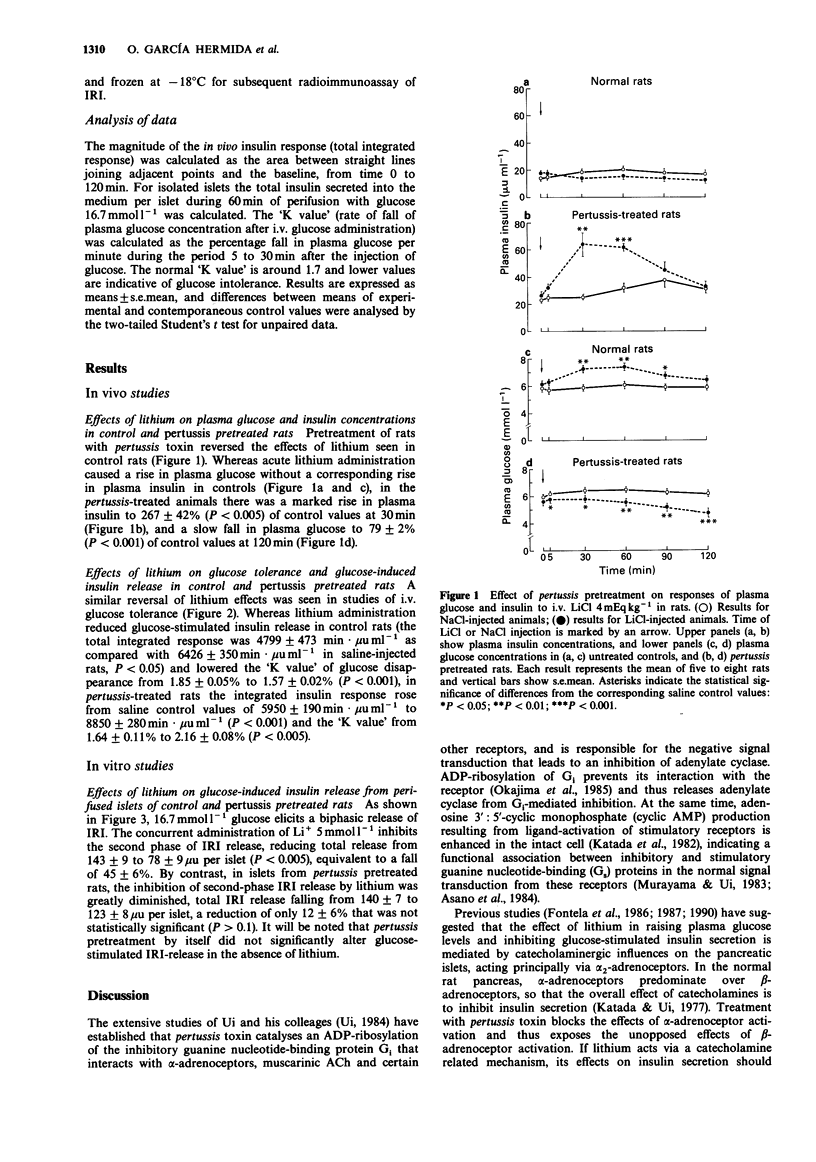
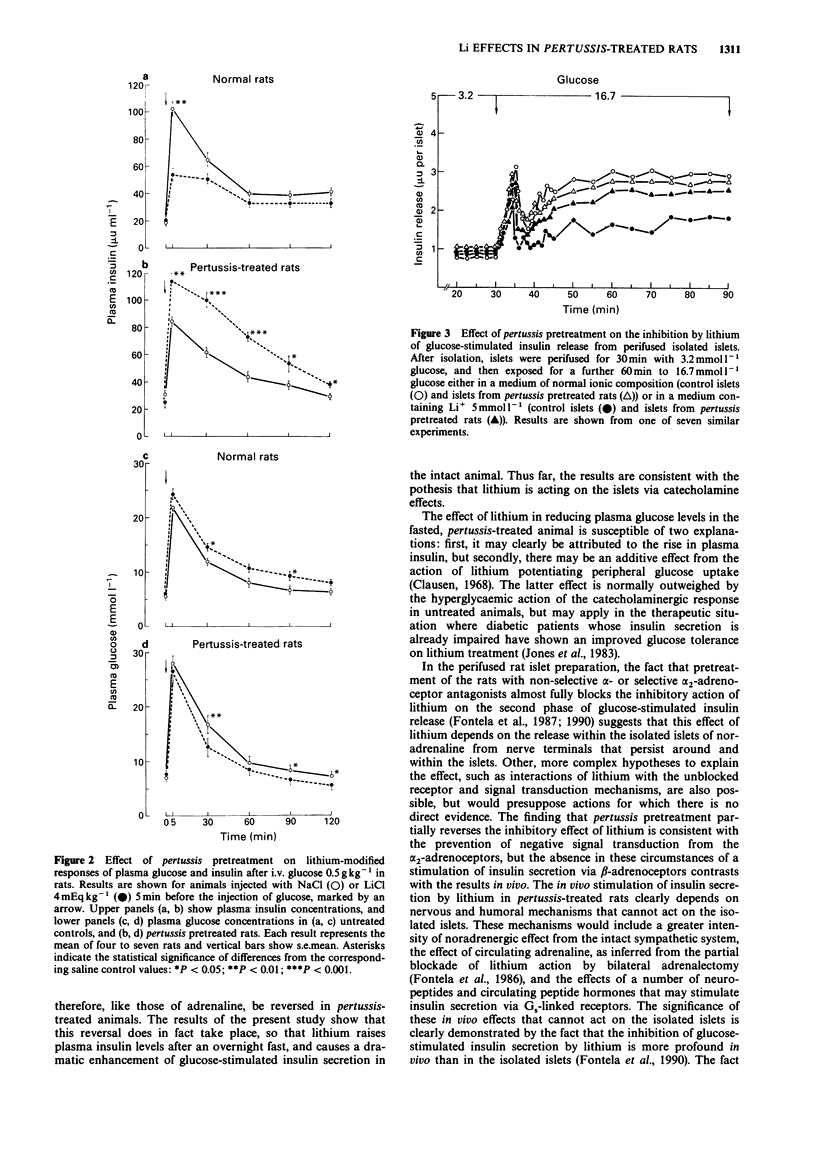
1. Administration of lithium to rats causes a rise in plasma glucose and suppresses glucose-stimulated insulin secretion. These effects are blocked by the alpha 2-adrenoceptor antagonist, yohimbine. 2. Pretreatment of rats with Bordetella pertussis toxin resulted in a reversal of the usual plasma glucose and insulin responses to intravenously administered lithium (4 mEq kg-1). There was a slow fall in plasma glucose, while plasma insulin rose to 267 +/- 42% (+/- s.e.mean) of control values at 30 min. The effect of lithium on glucose-stimulated insulin secretion was also reversed; there was a marked increase in the insulin response which contrasted with the suppression seen in normal controls. 3. In perifused islets of Langerhans isolated from pertussis pretreated rats, the previously described inhibition by lithium of the second phase of glucose-stimulated insulin secretion from normal islets was almost completely abolished. 4. The results are consistent with the hypothesis that these effects of lithium are mediated by the influence of catecholamines on the islets. When the inhibitory effect of alpha 2-adrenoceptors is abolished by pertussis treatment, which blocks the action of the inhibitory guanine nucleotide-binding protein Gi, effects of beta-adrenoceptor stimulation predominate, leading to an increased secretion of insulin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. H., Jr, Blackard W. G. Effect of lithium on pancreatic islet insulin release. Endocrinology. 1978 Jan;102(1):291–295. doi: 10.1210/endo-102-1-291. [DOI] [PubMed] [Google Scholar]
- Asano T., Katada T., Gilman A. G., Ross E. M. Activation of the inhibitory GTP-binding protein of adenylate cyclase, Gi, by beta-adrenergic receptors in reconstituted phospholipid vesicles. J Biol Chem. 1984 Aug 10;259(15):9351–9354. [PubMed] [Google Scholar]
- Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. Biochim Biophys Acta. 1968 Jan 3;150(1):66–72. doi: 10.1016/0005-2736(68)90009-6. [DOI] [PubMed] [Google Scholar]
- Fontela T., Garcia Hermida O., Gómez-Acebo J. Dihydroergotamine, but not naloxone, counteracts lithium as an inhibitor of glucose-induced insulin release in isolated rat islets in vitro. Diabetologia. 1987 Mar;30(3):183–187. doi: 10.1007/BF00274225. [DOI] [PubMed] [Google Scholar]
- Fontela T., García Hermida O., Gómez-Acebo J. Blocking effect of naloxone, dihydroergotamine and adrenalectomy in lithium-induced hyperglycaemia and glucose intolerance in the rat. Acta Endocrinol (Copenh) 1986 Mar;111(3):342–348. doi: 10.1530/acta.0.1110342. [DOI] [PubMed] [Google Scholar]
- Fontela T., García Hermida O., Gómez-Acebo J. Role of adrenoceptors in vitro and in vivo in the effects of lithium on blood glucose levels and insulin secretion in the rat. Br J Pharmacol. 1990 Jun;100(2):283–288. doi: 10.1111/j.1476-5381.1990.tb15796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. R., Lazarus J. H., Davies C. J., Greenwood R. H. The effect of short term lithium carbonate in Type II diabetes mellitus. Horm Metab Res. 1983 Sep;15(9):422–424. doi: 10.1055/s-2007-1018745. [DOI] [PubMed] [Google Scholar]
- Katada T., Amano T., Ui M. Modulation by islet-activating protein of adenylate cyclase activity in C6 glioma cells. J Biol Chem. 1982 Apr 10;257(7):3739–3746. [PubMed] [Google Scholar]
- Katada T., Ui M. Perfusion of the pancreas isolated from pertussis-sensitized rats: potentiation of insulin secretory responses due to beta-adrenergic stimulation. Endocrinology. 1977 Oct;101(4):1247–1255. doi: 10.1210/endo-101-4-1247. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Okajima F., Katada T., Ui M. Coupling of the guanine nucleotide regulatory protein to chemotactic peptide receptors in neutrophil membranes and its uncoupling by islet-activating protein, pertussis toxin. A possible role of the toxin substrate in Ca2+-mobilizing receptor-mediated signal transduction. J Biol Chem. 1985 Jun 10;260(11):6761–6768. [PubMed] [Google Scholar]
- Shah J. H., Pishdad G. The effect of lithium on glucose- and tolbutamide-induced insulin release and glucose tolerance in the intact rat. Endocrinology. 1980 Nov;107(5):1300–1304. doi: 10.1210/endo-107-5-1300. [DOI] [PubMed] [Google Scholar]
- Yamazaki S., Katada T., Ui M. Alpha 2-adrenergic inhibition of insulin secretion via interference with cyclic AMP generation in rat pancreatic islets. Mol Pharmacol. 1982 May;21(3):648–653. [PubMed] [Google Scholar]


