Abstract
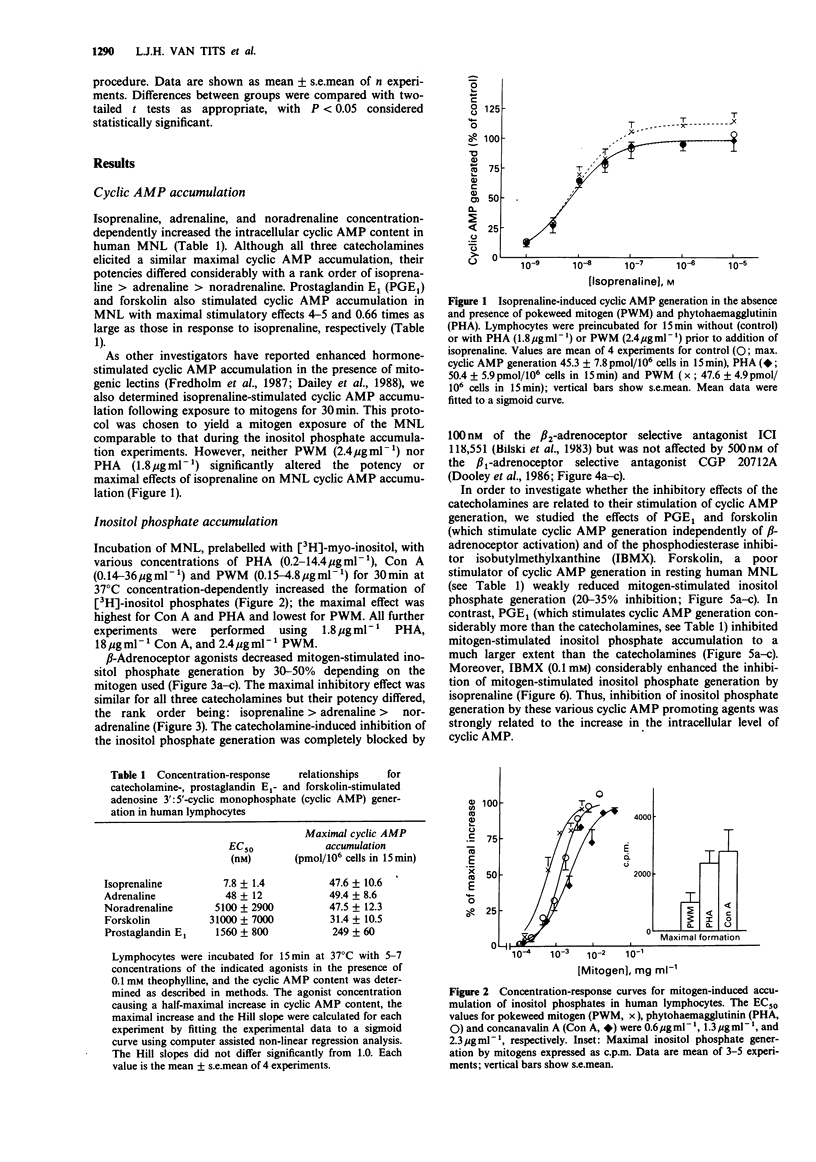
1. The effects of increases in intracellular adenosine 3':5'-cyclic monophosphate (cyclic AMP) on mitogen-induced generation of inositol phosphates and increases in intracellular Ca2+ concentration were investigated in human peripheral blood mononuclear leukocytes (MNL). 2. The mitogens concanavalin A (Con A), pokeweed mitogen (PWM) and phytohaemagglutinin (PHA) concentration-dependently stimulated generation of inositol phosphates. Catecholamines inhibited this process with an order of potency: isoprenaline greater than adrenaline greater than noradrenaline indicating involvement of beta 2-adrenoceptors. This order of potency was also consistent with the catecholamine potencies for stimulating the generation of cyclic AMP. 3. In addition to catecholamines, the cyclic AMP formation-stimulating agents prostaglandin E1 (PGE1) and forskolin concentration-dependently inhibited mitogen-induced inositol phosphate generation, too. Moreover, the inhibitory effect of isoprenaline was potentiated by co-incubation with the phosphodiesterase inhibitor isobutylmethylxanthine demonstrating that these inhibitory effects were mediated by cyclic AMP. 4. Con A and PHA concentration-dependently increased the intracellular Ca2+ concentration in human MNL (assessed by the fluorescent indicator dye Fura-2). This increase was almost completely blocked by chelation of extracellular Ca2+, demonstrating influx rather than mobilization from intracellular stores. 5. The elevation of intracellular Ca2+ was not blocked by pretreatment with pertussis toxin, 100 ng ml-1, for 16 h. 6. Isoprenaline, PGE1, and forskolin, however, inhibited the mitogen-stimulated elevation of intracellular Ca2+. This inhibition was enhanced by the phosphodiesterase inhibitors isobutylmethylxanthine and Ro 20-1724, demonstrating mediation by cyclic AMP. 7. We conclude that catecholamines and other cyclic AMP increasing agents can inhibit mitogen-stimulated generation of inositol phosphates and elevation of intracellular Ca2+ in resting human MNL.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

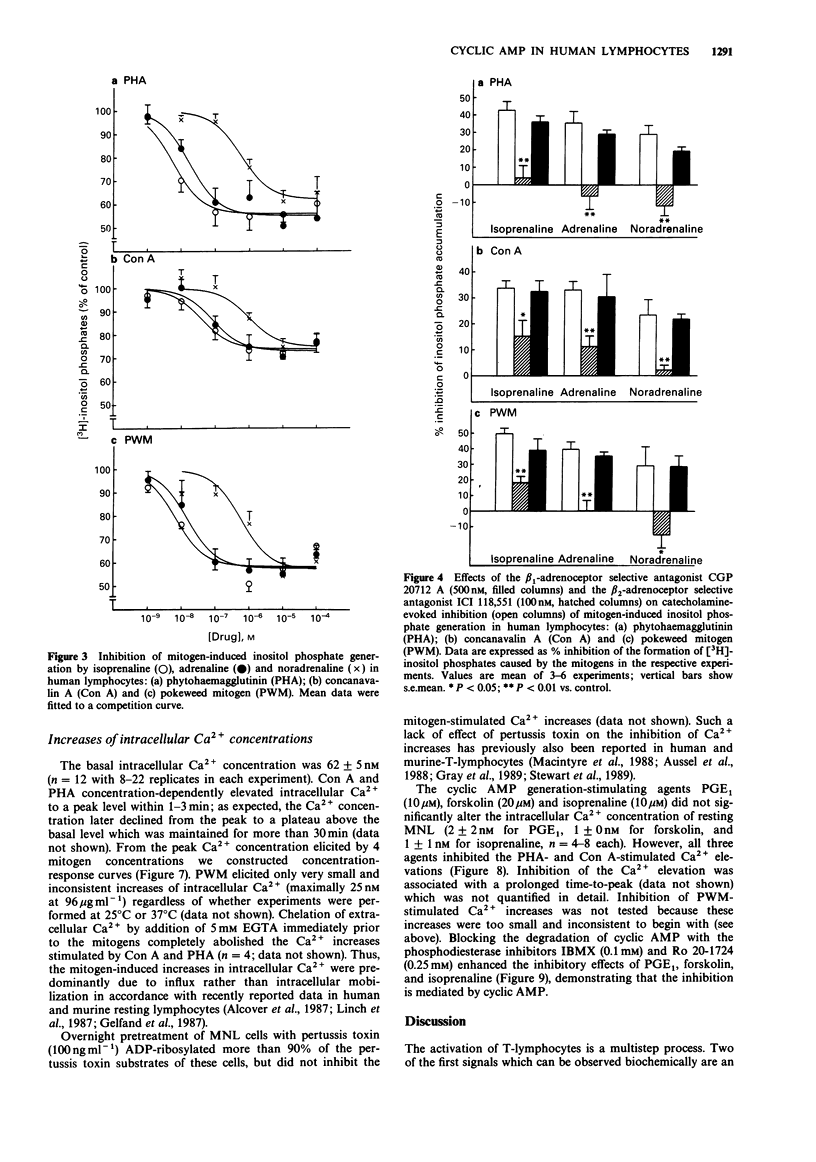

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Ramarli D., Richardson N. E., Chang H. C., Reinherz E. L. Functional and molecular aspects of human T lymphocyte activation via T3-Ti and T11 pathways. Immunol Rev. 1987 Feb;95:5–36. doi: 10.1111/j.1600-065x.1987.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Aussel C., Mary D., Peyron J. F., Pelassy C., Ferrua B., Fehlmann M. Inhibition and activation of interleukin 2 synthesis by direct modification of guanosine triphosphate-binding proteins. J Immunol. 1988 Jan 1;140(1):215–220. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bilski A. J., Halliday S. E., Fitzgerald J. D., Wale J. L. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551). J Cardiovasc Pharmacol. 1983 May-Jun;5(3):430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
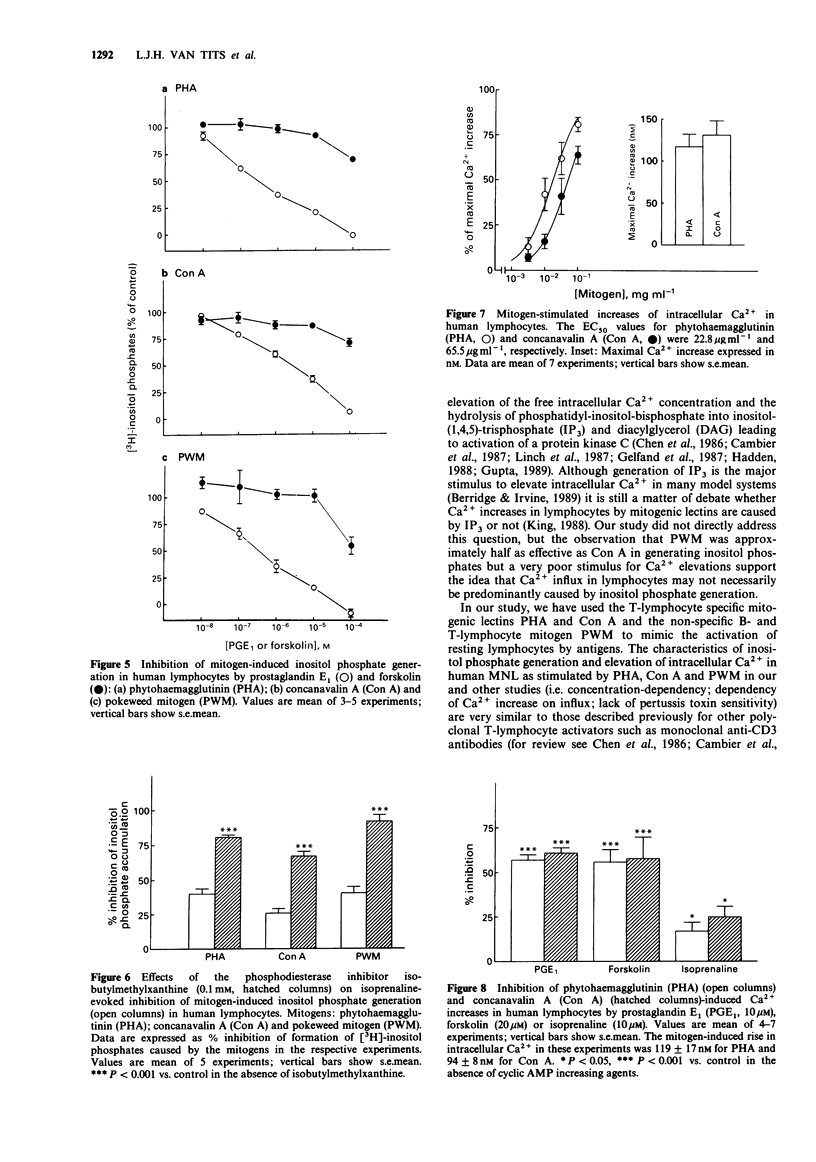
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Brodde O. E., Brinkmann M., Schemuth R., O'Hara N., Daul A. Terbutaline-induced desensitization of human lymphocyte beta 2-adrenoceptors. Accelerated restoration of beta-adrenoceptor responsiveness by prednisone and ketotifen. J Clin Invest. 1985 Sep;76(3):1096–1101. doi: 10.1172/JCI112063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cambier J. C., Justement L. B., Newell M. K., Chen Z. Z., Harris L. K., Sandoval V. M., Klemsz M. J., Ransom J. T. Transmembrane signals and intracellular "second messengers" in the regulation of quiescent B-lymphocyte activation. Immunol Rev. 1987 Feb;95:37–57. doi: 10.1111/j.1600-065x.1987.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Chen Z. Z., Coggeshall K. M., Cambier J. C. Translocation of protein kinase C during membrane immunoglobulin-mediated transmembrane signaling in B lymphocytes. J Immunol. 1986 Mar 15;136(6):2300–2304. [PubMed] [Google Scholar]
- Dailey M. O., Schreurs J., Schulman H. Hormone receptors on cloned T lymphocytes. Increased responsiveness to histamine, prostaglandins, and beta-adrenergic agents as a late stage event in T cell activation. J Immunol. 1988 May 1;140(9):2931–2936. [PubMed] [Google Scholar]
- Dooley D. J., Bittiger H., Reymann N. C. CGP 20712 A: a useful tool for quantitating beta 1- and beta 2-adrenoceptors. Eur J Pharmacol. 1986 Oct 14;130(1-2):137–139. doi: 10.1016/0014-2999(86)90193-7. [DOI] [PubMed] [Google Scholar]
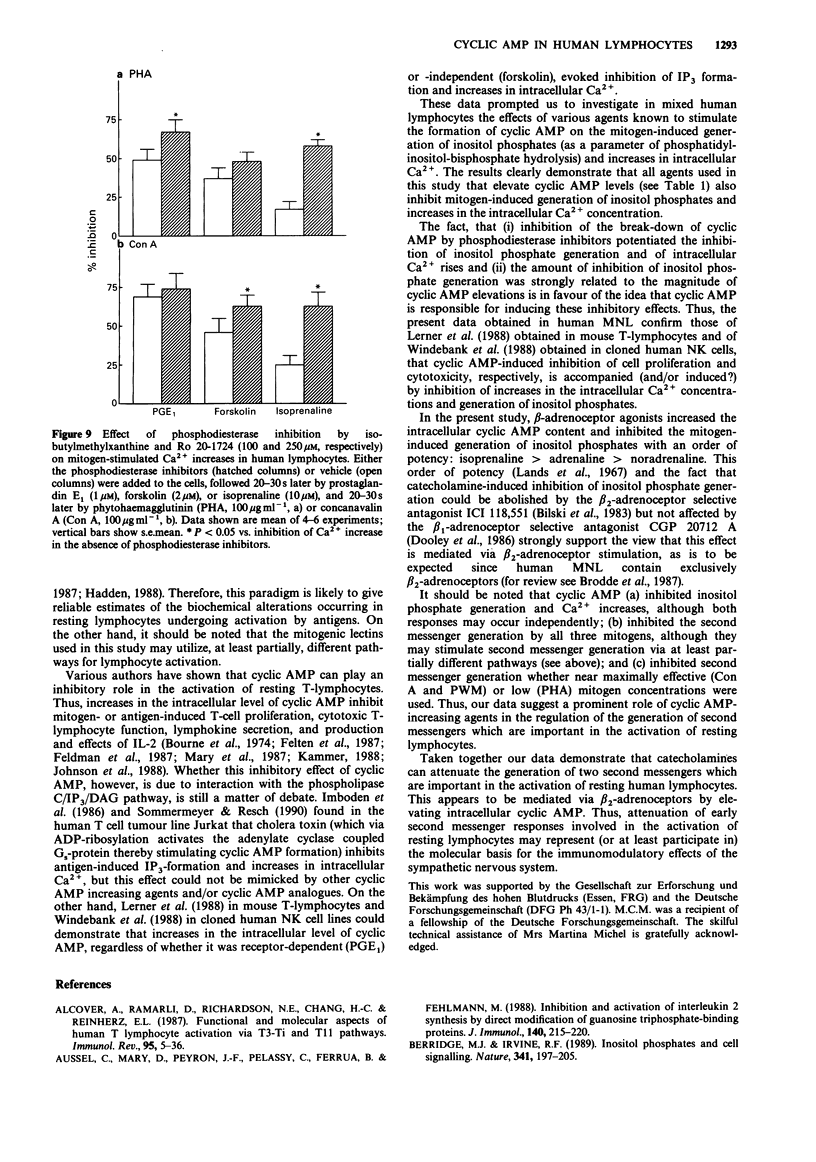
- Durant S. In vivo effects of catecholamines and glucocorticoids on mouse thymic cAMP content and thymolysis. Cell Immunol. 1986 Oct 1;102(1):136–143. doi: 10.1016/0008-8749(86)90332-1. [DOI] [PubMed] [Google Scholar]
- Feldman R. D., Hunninghake G. W., McArdle W. L. Beta-adrenergic-receptor-mediated suppression of interleukin 2 receptors in human lymphocytes. J Immunol. 1987 Nov 15;139(10):3355–3359. [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Bellinger D. L., Carlson S. L., Ackerman K. D., Madden K. S., Olschowki J. A., Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987 Dec;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Jondal M., Nordstedt C. The adenosine receptor mediated accumulation of cyclic AMP in Jurkat cells is enhanced by a lectin and by phorbol esters. Biochem Biophys Res Commun. 1987 May 29;145(1):344–349. doi: 10.1016/0006-291x(87)91327-1. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Mills G. B., Cheung R. K., Lee J. W., Grinstein S. Transmembrane ion fluxes during activation of human T lymphocytes: role of Ca2+, Na+/H+ exchange and phospholipid turnover. Immunol Rev. 1987 Feb;95:59–87. doi: 10.1111/j.1600-065x.1987.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Gray L. S., Huber K. S., Gray M. C., Hewlett E. L., Engelhard V. H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989 Mar 1;142(5):1631–1638. [PubMed] [Google Scholar]
- Gupta S. Mechanisms of transmembrane signalling in human T cell activation. 1989 Nov 23-Dec 19Mol Cell Biochem. 91(1-2):45–50. doi: 10.1007/BF00228078. [DOI] [PubMed] [Google Scholar]
- Hadden J. W. Transmembrane signals in the activation of T lymphocytes by mitogenic antigens. Immunol Today. 1988 Jul-Aug;9(7-8):235–239. doi: 10.1016/0167-5699(88)91222-4. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. W., Davis B. H., Smith K. A. cAMP antagonizes interleukin 2-promoted T-cell cycle progression at a discrete point in early G1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6072–6076. doi: 10.1073/pnas.85.16.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- King S. L. An assessment of phosphoinositide hydrolysis in antigenic signal transduction in lymphocytes. Immunology. 1988 Sep;65(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Lerner A., Jacobson B., Miller R. A. Cyclic AMP concentrations modulate both calcium flux and hydrolysis of phosphatidylinositol phosphates in mouse T lymphocytes. J Immunol. 1988 Feb 1;140(3):936–940. [PubMed] [Google Scholar]
- Linch D. C., Wallace D. L., O'Flynn K. Signal transduction in human T lymphocytes. Immunol Rev. 1987 Feb;95:137–159. doi: 10.1111/j.1600-065x.1987.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Macintyre E. A., Tatham P. E., Abdul-Gaffar R., Linch D. C. The effects of pertussis toxin on human T lymphocytes. Immunology. 1988 Jul;64(3):427–432. [PMC free article] [PubMed] [Google Scholar]
- Maisel A. S., Knowlton K. U., Fowler P., Rearden A., Ziegler M. G., Motulsky H. J., Insel P. A., Michel M. C. Adrenergic control of circulating lymphocyte subpopulations. Effects of congestive heart failure, dynamic exercise, and terbutaline treatment. J Clin Invest. 1990 Feb;85(2):462–467. doi: 10.1172/JCI114460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneman K. P., Johnson R. D. Characterization of alpha-1 adrenergic receptors linked to [3H]inositol metabolism in rat cerebral cortex. J Pharmacol Exp Ther. 1984 Aug;230(2):317–323. [PubMed] [Google Scholar]
- Motulsky H. J., Michel M. C. Neuropeptide Y mobilizes Ca2+ and inhibits adenylate cyclase in human erythroleukemia cells. Am J Physiol. 1988 Dec;255(6 Pt 1):E880–E885. doi: 10.1152/ajpendo.1988.255.6.E880. [DOI] [PubMed] [Google Scholar]
- Sommermeyer H., Resch K. Pertussis toxin B-subunit-induced Ca2(+)-fluxes in Jurkat human lymphoma cells: the action of long-term pre-treatment with cholera and pertussis holotoxins. Cell Signal. 1990;2(2):115–128. doi: 10.1016/0898-6568(90)90015-3. [DOI] [PubMed] [Google Scholar]
- Stewart S. J., Prpic V., Johns J. A., Powers F. S., Graber S. E., Forbes J. T., Exton J. H. Bacterial toxins affect early events of T lymphocyte activation. J Clin Invest. 1989 Jan;83(1):234–242. doi: 10.1172/JCI113865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T., Waku K. Enhanced turnover of arachidonic acid-containing species of phosphatidylinositol and phosphatidic acid of concanavalin A-stimulated lymphocytes. Biochim Biophys Acta. 1984 Nov 14;796(2):190–198. [PubMed] [Google Scholar]
- Taylor M. V., Metcalfe J. C., Hesketh T. R., Smith G. A., Moore J. P. Mitogens increase phosphorylation of phosphoinositides in thymocytes. 1984 Nov 29-Dec 5Nature. 312(5993):462–465. doi: 10.1038/312462a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Windebank K. P., Abraham R. T., Powis G., Olsen R. A., Barna T. J., Leibson P. J. Signal transduction during human natural killer cell activation: inositol phosphate generation and regulation by cyclic AMP. J Immunol. 1988 Dec 1;141(11):3951–3957. [PubMed] [Google Scholar]
- Wolff C. H., Akerman K. E. Concanavalin A binding and Ca2+ fluxes in rat spleen cells. Biochim Biophys Acta. 1982 Dec 22;693(2):315–319. doi: 10.1016/0005-2736(82)90437-0. [DOI] [PubMed] [Google Scholar]


