Abstract
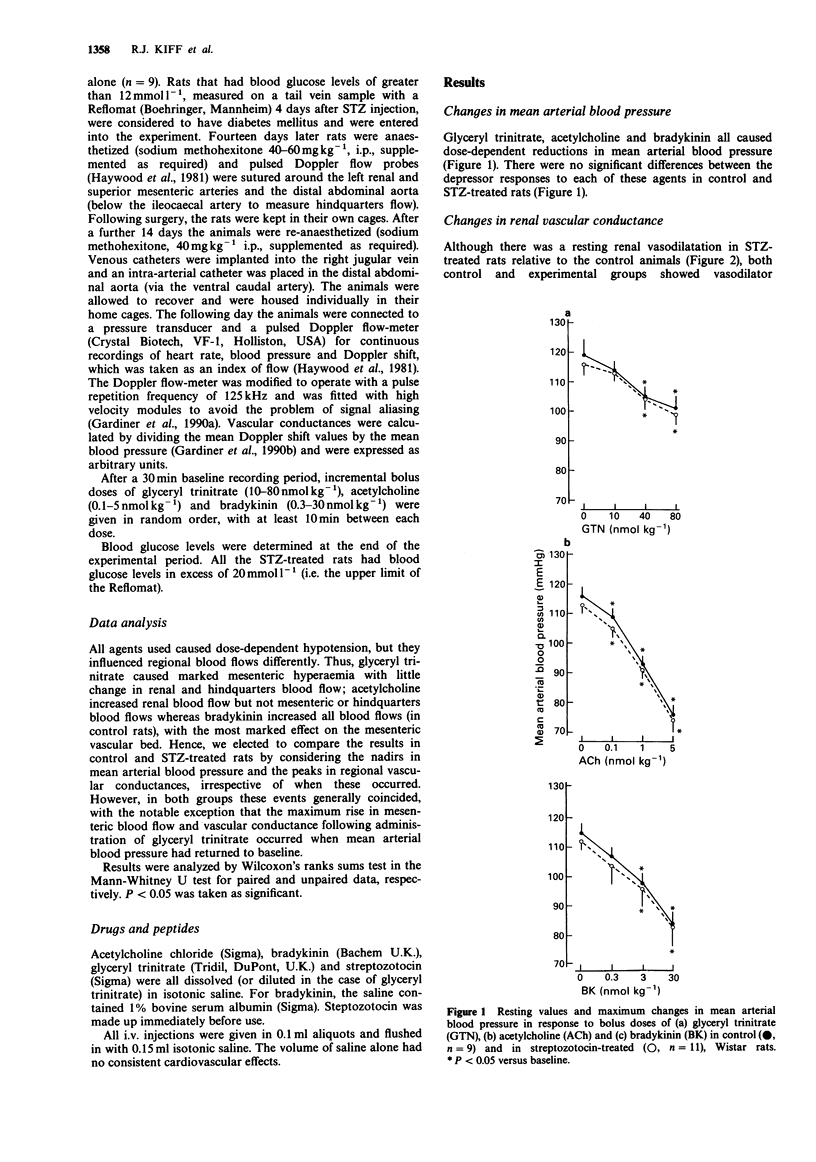
1. Male, Wistar rats were treated with streptozotocin (STZ, 70 mg kg-1, i.p.) or saline and chronically instrumented with pulsed Doppler probes and intravascular catheters (implanted under sodium methohexitone anaesthesia) to allow assessment of haemodynamics in the conscious state 28 days later. 2. Control and STZ-treated rats received bolus doses of glyceryl trinitrate (10-80 nmol kg-1), acetylcholine (0.1-5 nmol kg-1) and bradykinin (0.3-30 nmol kg-1). 3. Although, as reported previously, STZ-treated rats had normal mean arterial blood pressure together with renal and mesenteric vasodilatations and hindquarters vasoconstriction relative to control rats, both groups showed similar hypotensive and regional haemodynamic responses to glyceryl trinitrate and acetylcholine. However, while the depressor effects of bradykinin were similar in control and STZ-treated rats, the former showed a hindquarters vasodilator response to bradykinin that was absent in the STZ-treated rats. 4. A loss of bradykinin-mediated vasodilatation in the hindquarters vascular bed in STZ-treated rats in the presence of normal, hindquarters vasodilator responses to other agents and normal bradykinin-mediated vasodilator responses in other vascular beds is consistent with existing evidence that the vasodilatation elicited by bradykinin in the hindquarters vascular bed is particularly dependent on nitric oxide synthesis and that this is impaired selectively in STZ-treated rats.
Full text
PDF
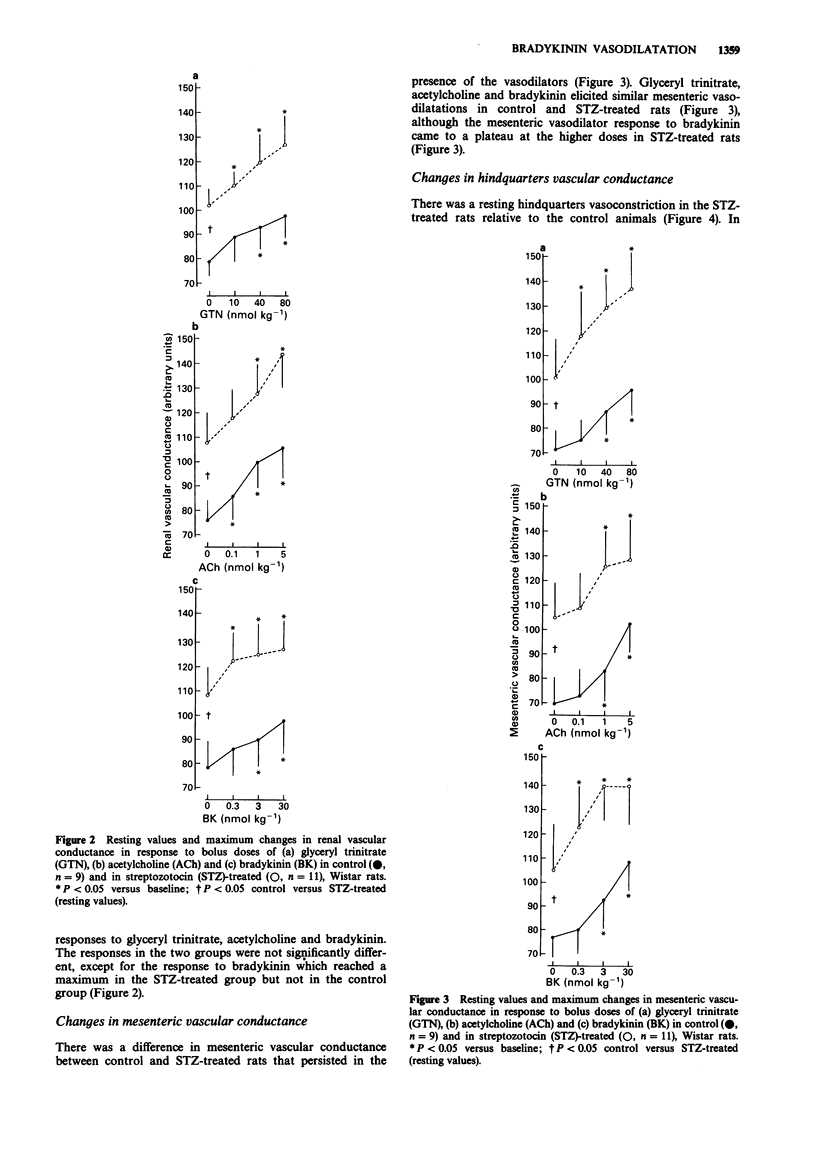
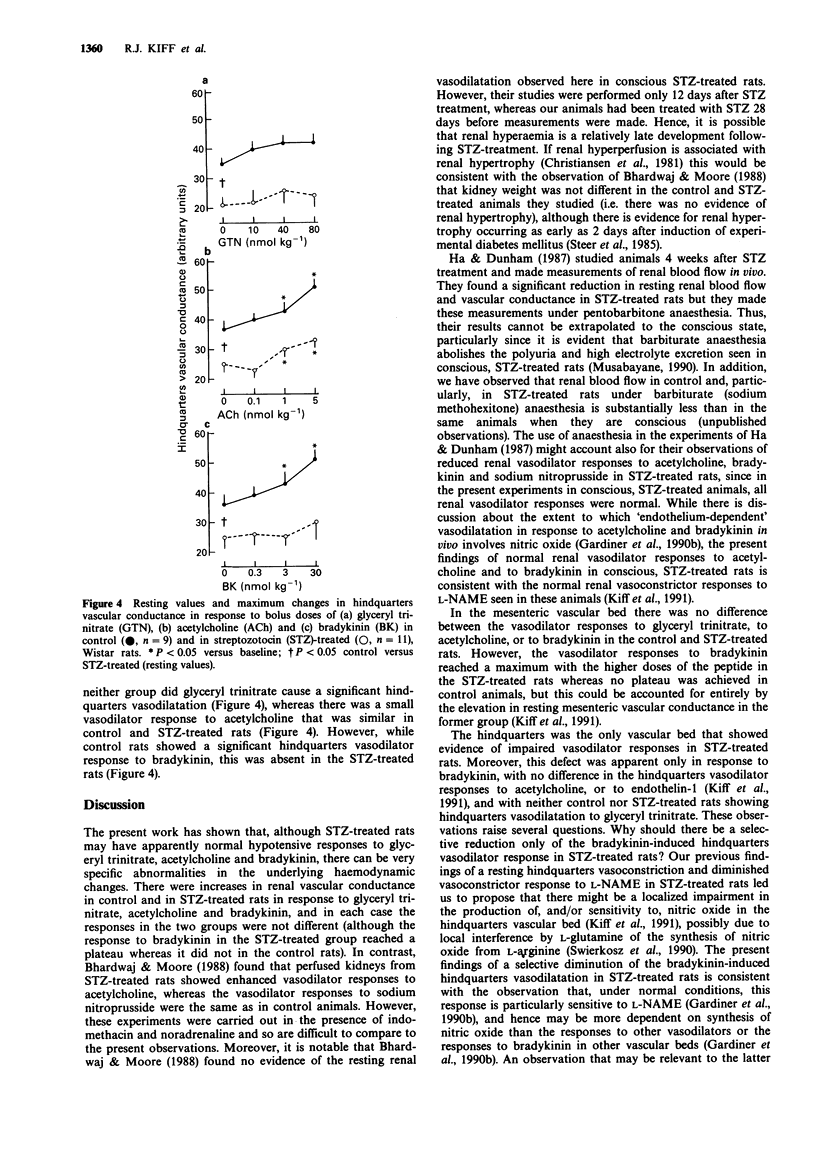


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- Altan V. M., Karasu C., Ozüari A. The effects of type-1 and type-2 diabetes on endothelium-dependent relaxation in rat aorta. Pharmacol Biochem Behav. 1989 Jul;33(3):519–522. doi: 10.1016/0091-3057(89)90379-1. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R., Moore P. K. Increased vasodilator response to acetylcholine of renal blood vessels from diabetic rats. J Pharm Pharmacol. 1988 Oct;40(10):739–742. doi: 10.1111/j.2042-7158.1988.tb07009.x. [DOI] [PubMed] [Google Scholar]
- Christiansen J. S., Gammelgaard J., Frandsen M., Parving H. H. Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetologia. 1981 Apr;20(4):451–456. doi: 10.1007/BF00253406. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Hartley C. J. Can pulsed Doppler technique measure changes in aortic blood flow in conscious rats? Am J Physiol. 1990 Aug;259(2 Pt 2):H448–H456. doi: 10.1152/ajpheart.1990.259.2.H448. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Botting R. M., Vane J. R. Mediators produced by the endothelial cell. Hypertension. 1988 Dec;12(6):530–548. doi: 10.1161/01.hyp.12.6.530. [DOI] [PubMed] [Google Scholar]
- Ha H., Dunham E. W. Limited capacity for renal vasodilatation in anesthetized diabetic rats. Am J Physiol. 1987 Oct;253(4 Pt 2):H845–H855. doi: 10.1152/ajpheart.1987.253.4.H845. [DOI] [PubMed] [Google Scholar]
- Harris K. H., MacLeod K. M. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur J Pharmacol. 1988 Aug 9;153(1):55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- Haywood J. R., Shaffer R. A., Fastenow C., Fink G. D., Brody M. J. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rat. Am J Physiol. 1981 Aug;241(2):H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- Head R. J., Longhurst P. A., Panek R. L., Stitzel R. E. A contrasting effect of the diabetic state upon the contractile responses of aortic preparations from the rat and rabbit. Br J Pharmacol. 1987 Jun;91(2):275–286. doi: 10.1111/j.1476-5381.1987.tb10282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebden R. A., Gardiner S. M., Bennett T., MacDonald I. A. The influence of streptozotocin-induced diabetes mellitus on fluid and electrolyte handling in rats. Clin Sci (Lond) 1986 Jan;70(1):111–117. doi: 10.1042/cs0700111. [DOI] [PubMed] [Google Scholar]
- Kaiser L., Sparks H. V., Jr Endothelial cells. Not just a cellophane wrapper. Arch Intern Med. 1987 Mar;147(3):569–573. doi: 10.1001/archinte.147.3.569. [DOI] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989 Jun;97(2):614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto J. H., McLaughlin B. E., Brien J. F., Marks G. S., Nakatsu K. Biotransformation of glyceryl trinitrate and elevation of cyclic GMP precede glyceryl trinitrate-induced vasodilation. J Cardiovasc Pharmacol. 1990 May;15(5):714–719. doi: 10.1097/00005344-199005000-00005. [DOI] [PubMed] [Google Scholar]
- Long C. J., Berkowitz B. A. What is the relationship between the endothelium derived relaxant factor and nitric oxide? Life Sci. 1989;45(1):1–14. doi: 10.1016/0024-3205(89)90429-3. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G. Impairment of endothelium-dependent dilatation of cerebral arterioles during diabetes mellitus. Am J Physiol. 1989 Mar;256(3 Pt 2):H621–H625. doi: 10.1152/ajpheart.1989.256.3.H621. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension. 1988 Oct;12(4):365–372. doi: 10.1161/01.hyp.12.4.365. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern M., Docherty J. R. Effects of experimental diabetes on the responsiveness of rat aorta. Br J Pharmacol. 1989 Aug;97(4):1007–1012. doi: 10.1111/j.1476-5381.1989.tb12555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Pieper G. M., Gross G. J. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1988 Oct;255(4 Pt 2):H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- Porta M., La Selva M., Molinatti P., Molinatti G. M. Endothelial cell function in diabetic microangiopathy. Diabetologia. 1987 Aug;30(8):601–609. doi: 10.1007/BF00277315. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer K. A., Sochor M., McLean P. Renal hypertrophy in experimental diabetes. Changes in pentose phosphate pathway activity. Diabetes. 1985 May;34(5):485–490. doi: 10.2337/diab.34.5.485. [DOI] [PubMed] [Google Scholar]
- Swierkosz T. A., Mitchell J. A., Sessa W. C., Hecker M., Vane J. R. L-glutamine inhibits the release of endothelium-derived relaxing factor from the rabbit aorta. Biochem Biophys Res Commun. 1990 Oct 15;172(1):143–148. doi: 10.1016/s0006-291x(05)80184-6. [DOI] [PubMed] [Google Scholar]
- Vatner S. F., Pagani M., Rutherford J. D., Millard R. W., Manders W. T. Effects of nitroglycerin on cardiac function and regional blood flow distribution in conscious dogs. Am J Physiol. 1978 Mar;234(3):H244–H252. doi: 10.1152/ajpheart.1978.234.3.H244. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I., Hatake K., Kimura N., Kakishita E., Nagai K. Modulation of vascular tonus by the endothelium in experimental diabetes. Life Sci. 1987 Feb 16;40(7):643–648. doi: 10.1016/0024-3205(87)90265-7. [DOI] [PubMed] [Google Scholar]


