Abstract
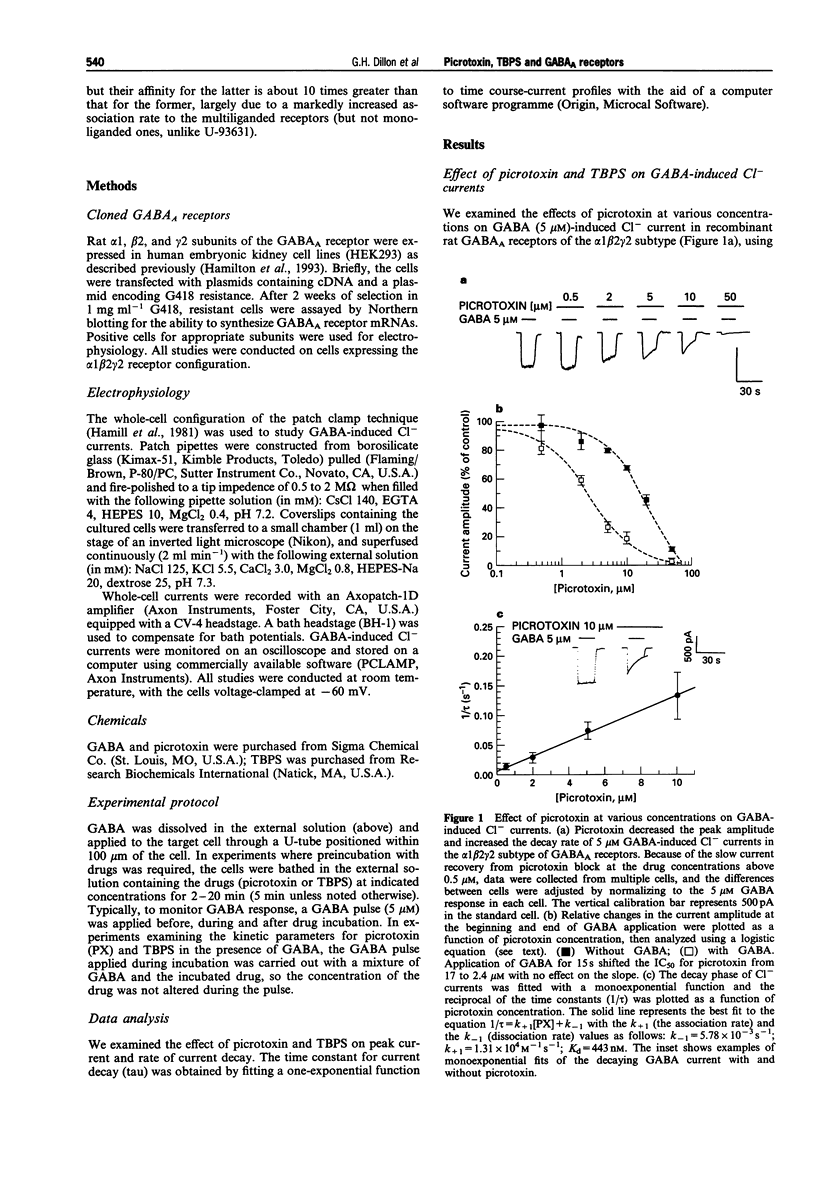
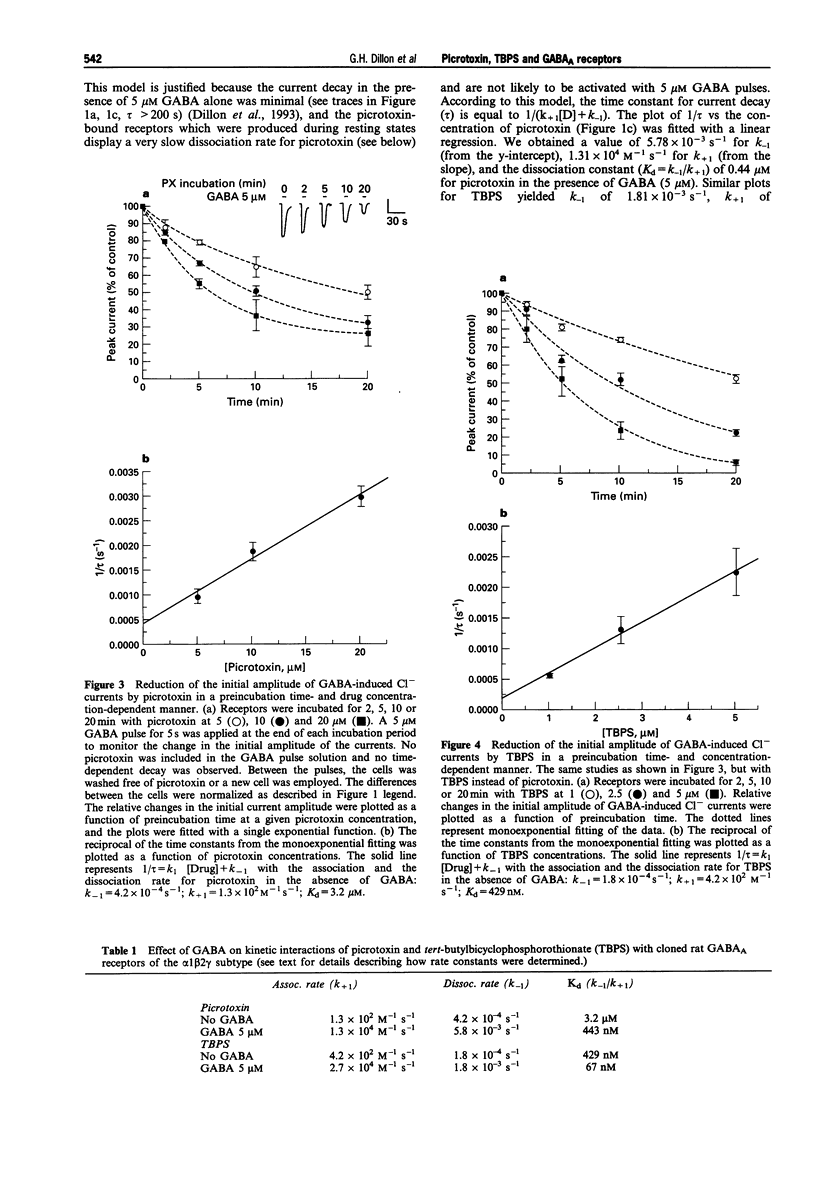
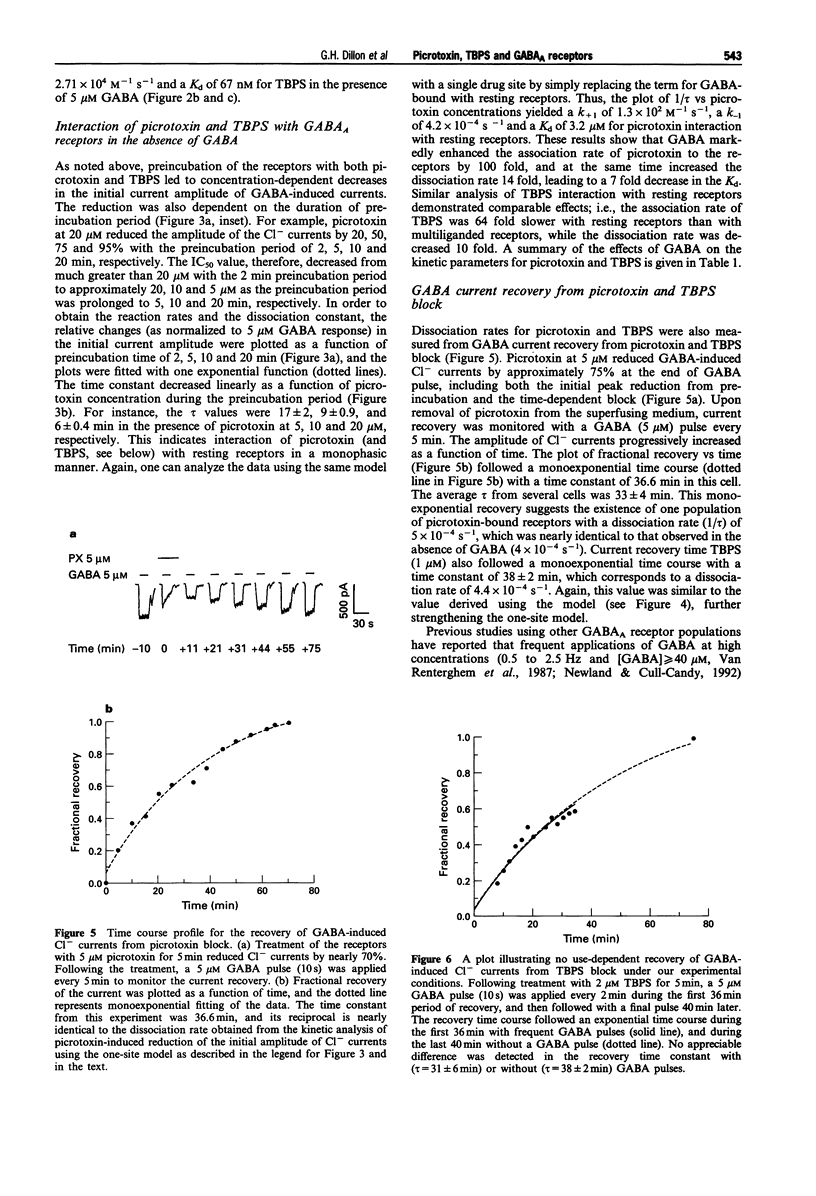
1. We examined how gamma-aminobutyric acid (GABA) influences interaction of picrotoxin and tert-butylbicyclophosphorothionate (TBPS) with recombinant rat alpha 1 beta 2 gamma 2 GABAA receptors stably expressed in human embryonic kidney cells (HEK293), as monitored with changes in Cl- currents measured by the whole-cell patch clamp technique. 2. During application of GABA (5 microM) for 15 s, picrotoxin and TBPS dose-dependently accelerated the decay of inward GABA-induced currents (a holding potential of -60 mV under a symmetrical Cl- gradient). The drugs, upon preincubation with the receptors, also reduced the initial current amplitude in a preincubation time and concentration-dependent manner. This indicates their interaction with both GABA-bound and resting receptors. 3. The half maximal inhibitory concentration for picrotoxin and TBPS at the beginning of a 15 s GABA (5 microM) pulse was several times greater than that obtained at the end of the pulse. GABA thus appears to enhance picrotoxin and TBPS potency, but only at concentrations leading to occupancy of both high and low affinity GABA sites, i.e., 5 microM. Preincubation of the receptors with the drugs in the presence of GABA at 200 nM, which leads to occupancy of only high affinity GABA sites in the alpha 1 beta 2 gamma 2 subtype, produced no appreciable change in potency of picrotoxin or TBPS. This indicates that they preferentially interact with multiliganded, but not monoliganded receptors, unlike U-93631, a novel ligand to the picrotoxin site, which has higher affinity to both mono- and multiliganded receptors than resting receptors.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Oomura Y., Carpenter D. O. Bicuculline and picrotoxin block gamma-aminobutyric acid-gated Cl- conductance by different mechanisms. Experientia. 1985 Jan 15;41(1):70–71. doi: 10.1007/BF02005880. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., Mathers D. A. Convulsant-induced depression of amino acid responses in cultured mouse spinal neurones studied under voltage clamp. Br J Pharmacol. 1983 Dec;80(4):619–629. doi: 10.1111/j.1476-5381.1983.tb10051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Collins J. F., Hill R. G. Bicyclic phosphorus esters that are potent convulsants and GABA antagonists. Nature. 1976 Jun 17;261(5561):601–603. doi: 10.1038/261601a0. [DOI] [PubMed] [Google Scholar]
- Constanti A. The "mixed" effect of picrotoxin on the GABA dose/conductance relation recorded from lobster muscle. Neuropharmacology. 1978 Mar;17(3):159–167. doi: 10.1016/0028-3908(78)90095-3. [DOI] [PubMed] [Google Scholar]
- Dillon G. H., Im H. K., Hamilton B. J., Carter D. B., Gammill R. B., Judge T. M., Im W. B. U-93631 causes rapid decay of gamma-aminobutyric acid-induced chloride currents in recombinant rat gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1993 Oct;44(4):860–865. [PubMed] [Google Scholar]
- Dillon G. H., Im W. B., Pregenzer J. F., Carter D. B., Hamilton B. J. [4-Dimethyl-3-t-butylcarboxyl-4,5-dihydro (1,5-a) quinoxaline] is a novel ligand to the picrotoxin site on GABAA receptors, and decreases single-channel open probability. J Pharmacol Exp Ther. 1995 Feb;272(2):597–603. [PubMed] [Google Scholar]
- Hamann M., Desarmenien M., Vanderheyden P., Piguet P., Feltz P. Electrophysiological study of tert-butylbicyclophosphorothionate-induced block of spontaneous chloride channels. Mol Pharmacol. 1990 Apr;37(4):578–582. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton B. J., Lennon D. J., Im H. K., Im W. B., Seeburg P. H., Carter D. B. Stable expression of cloned rat GABAA receptor subunits in a human kidney cell line. Neurosci Lett. 1993 Apr 30;153(2):206–209. doi: 10.1016/0304-3940(93)90323-d. [DOI] [PubMed] [Google Scholar]
- Newland C. F., Cull-Candy S. G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J Physiol. 1992 Feb;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregenzer J. F., Im W. B., Carter D. B., Thomsen D. R. Comparison of interactions of [3H]muscimol, t-butylbicyclophosphoro[35S]thionate, and [3H]flunitrazepam with cloned gamma-aminobutyric acidA receptors of the alpha 1 beta 2 and alpha 1 beta 2 gamma 2 subtypes. Mol Pharmacol. 1993 May;43(5):801–806. [PubMed] [Google Scholar]
- Ramanjaneyulu R., Ticku M. K. Binding characteristics and interactions of depressant drugs with [35S]t-butylbicyclophosphorothionate, a ligand that binds to the picrotoxinin site. J Neurochem. 1984 Jan;42(1):221–229. doi: 10.1111/j.1471-4159.1984.tb09721.x. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Casida J. E., Richardson M., Saederup E. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to gamma-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983 Mar;23(2):326–336. [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Renterghem C., Bilbe G., Moss S., Smart T. G., Constanti A., Brown D. A., Barnard E. A. GABA receptors induced in Xenopus oocytes by chick brain mRNA: evaluation of TBPS as a use-dependent channel-blocker. Brain Res. 1987 Apr;388(1):21–31. doi: 10.1016/0169-328x(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Woodward R. M., Polenzani L., Miledi R. Characterization of bicuculline/baclofen-insensitive gamma-aminobutyric acid receptors expressed in Xenopus oocytes. I. Effects of Cl- channel inhibitors. Mol Pharmacol. 1992 Jul;42(1):165–173. [PubMed] [Google Scholar]
- Yakushiji T., Tokutomi N., Akaike N., Carpenter D. O. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987 Sep;22(3):1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]


