Abstract
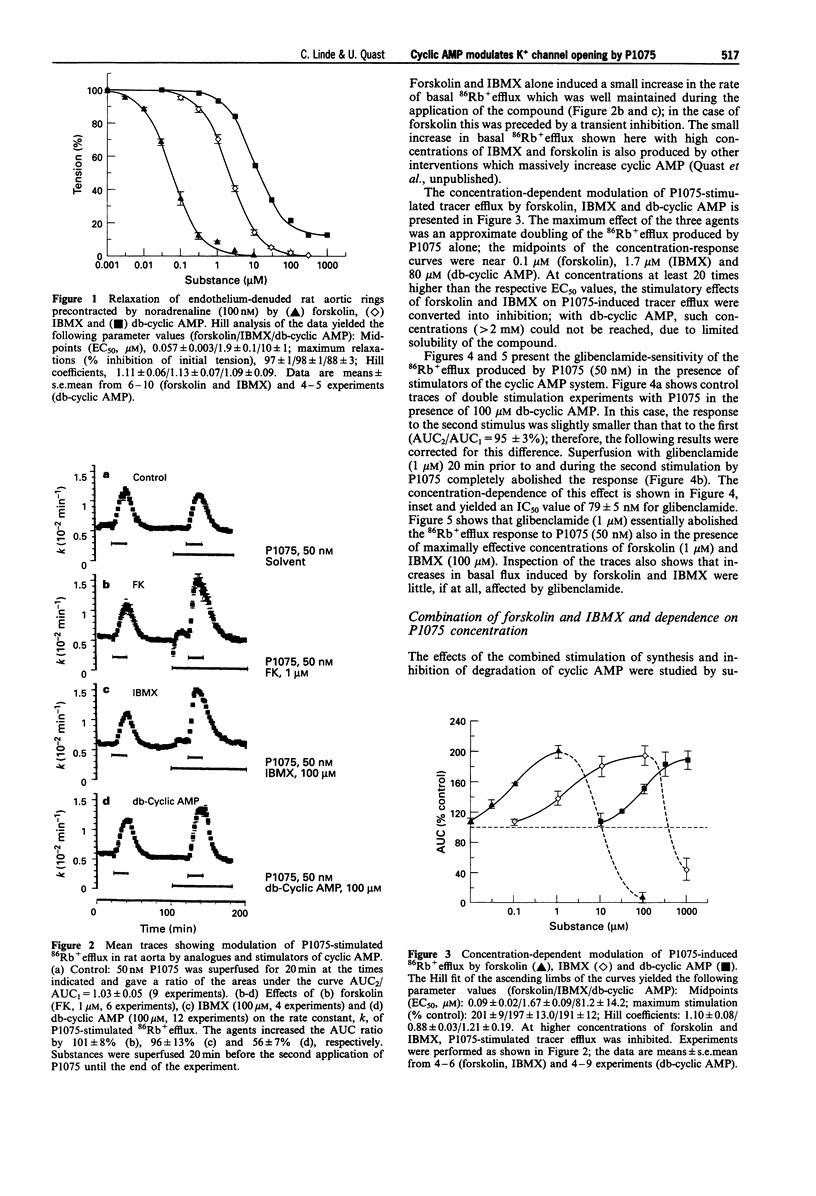
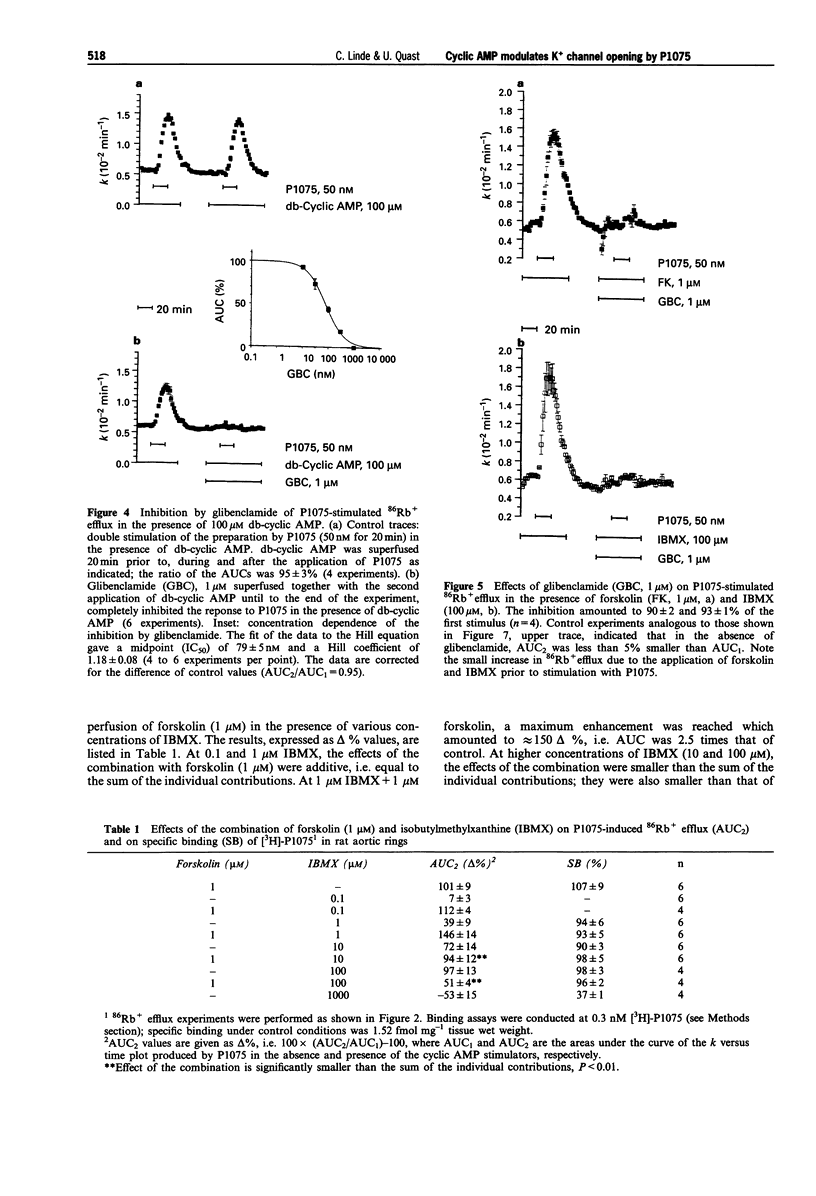
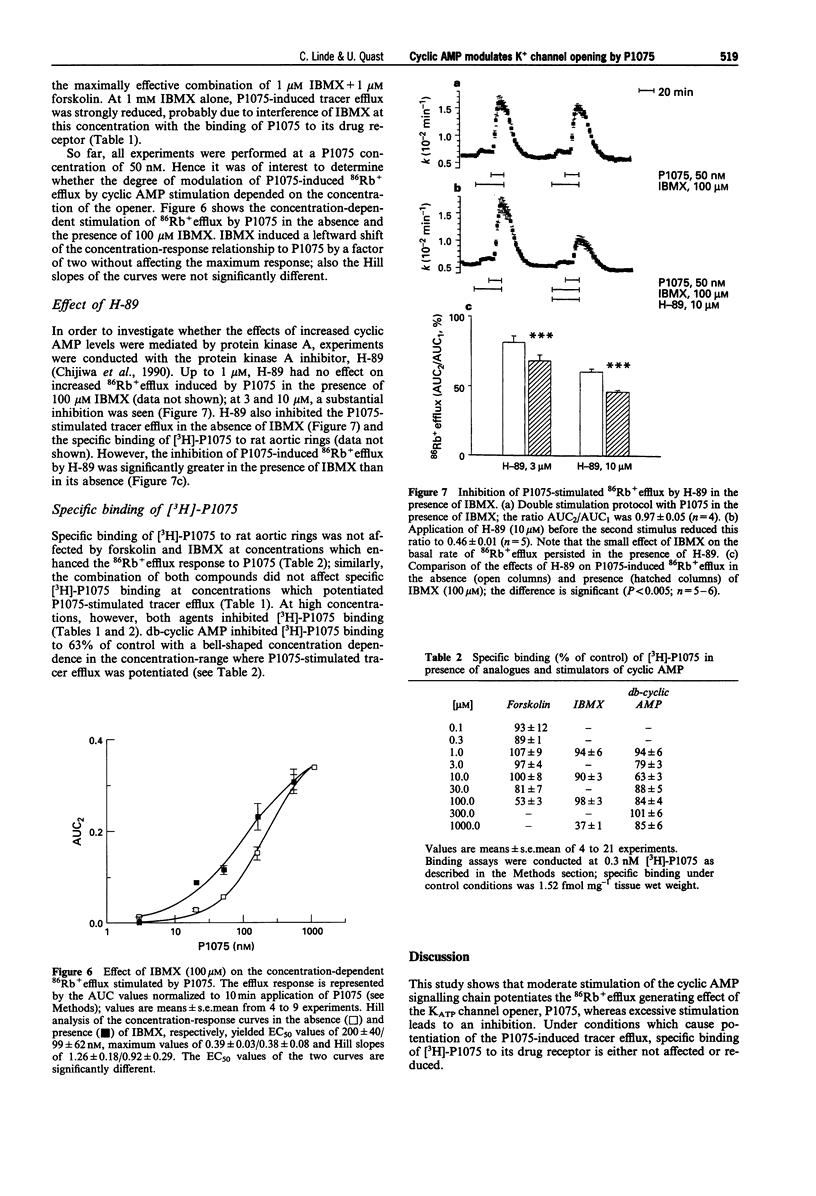
1. The effects of analogues and stimulators of cyclic AMP on the 86Rb+ efflux-stimulating and binding properties of P1075, an opener of ATP-dependent potassium channels, were studied in rat aortic rings. The increase in 86Rb+ efflux stimulated by P1075 was taken as a qualitative measure of K+ channel opening. 2. Forskolin, a direct activator of adenylate cyclase, isobutylmethylxanthine (IBMX), a phosphodiesterase inhibitor, and dibutyryl-cyclic AMP (db-cyclic AMP), a membrane permeant cyclic AMP-analogue, relaxed rat aortic rings contracted by noradrenaline with EC50 values of 0.06, 2 and 10 microM, respectively. 3. Forskolin, IBMX and db-cyclic AMP produced concentration-dependent increases of the 86Rb+ efflux induced by P1075 (50 nM) by up to twofold with EC50 values of about 0.1, 1.7 and 81 microM. At these concentrations the agents had little effect on the basal rate of 86Rb+ efflux. 4. The 86Rb+ efflux produced by P1075 in the presence of the cyclic AMP stimulators was inhibited by glibenclamide, a blocker of ATP-sensitive potassium channels. 5. IBMX (100 microM) induced a leftward shift of the concentration-86Rb+ efflux curve of P1075 without increasing the maximum. The enhancements of P1075-stimulated 86Rb+ efflux produced by combinations of forskolin and IBMX were either additive or less than additive. 6. The protein kinase A inhibitor, H-89, inhibited P1075-stimulated 86Rb+ efflux in the presence of IBMX significantly more than in the absence of IBMX, suggesting that the effect of increased cyclic AMP levels is mediated by protein kinase A.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Bond C. T., Blair T. A., Adelman J. P. Cloning and functional expression of a rat heart KATP channel. Nature. 1994 Aug 11;370(6489):456–459. doi: 10.1038/370456a0. [DOI] [PubMed] [Google Scholar]
- Bray K. M., Quast U. A specific binding site for K+ channel openers in rat aorta. J Biol Chem. 1992 Jun 15;267(17):11689–11692. [PubMed] [Google Scholar]
- Catterall W. A. The molecular basis of neuronal excitability. Science. 1984 Feb 17;223(4637):653–661. doi: 10.1126/science.6320365. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Coldwell M. C., Howlett D. R. Potassium efflux enhancement by cromakalim (BRL 34915) in rabbit mesenteric artery: an indirect effect independent of calcium? Biochem Pharmacol. 1988 Nov 1;37(21):4105–4110. doi: 10.1016/0006-2952(88)90102-5. [DOI] [PubMed] [Google Scholar]
- Edwards G., Ibbotson T., Weston A. H. Levcromakalim may induce a voltage-independent K-current in rat portal veins by modifying the gating properties of the delayed rectifier. Br J Pharmacol. 1993 Nov;110(3):1037–1048. doi: 10.1111/j.1476-5381.1993.tb13918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Induction of a glibenclamide-sensitive K-current by modification of a delayed rectifier channel in rat portal vein in insulinoma cells. Br J Pharmacol. 1993 Dec;110(4):1280–1281. doi: 10.1111/j.1476-5381.1993.tb13955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. KATP--fact or artefact? New thoughts on the mode of action of the potassium channel openers. Cardiovasc Res. 1994 Jun;28(6):735–745. doi: 10.1093/cvr/28.6.735. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R., Goll A., Rombusch M. Molecular pharmacology of the calcium channel: evidence for subtypes, multiple drug-receptor sites, channel subunits, and the development of a radioiodinated 1,4-dihydropyridine calcium channel label, [125I]iodipine. J Cardiovasc Pharmacol. 1984;6 (Suppl 4):S608–S621. [PubMed] [Google Scholar]
- Honoré E., Lazdunski M. Hormone-regulated K+ channels in follicle-enclosed oocytes are activated by vasorelaxing K+ channel openers and blocked by antidiabetic sulfonylureas. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5438–5442. doi: 10.1073/pnas.88.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Lazdunski M. Single-channel properties and regulation of pinacidil/glibenclamide-sensitive K+ channels in follicular cells from Xenopus oocyte. Pflugers Arch. 1993 Jul;424(2):113–121. doi: 10.1007/BF00374601. [DOI] [PubMed] [Google Scholar]
- Latorre R., Bacigalupo J., Delgado R., Labarca P. Four cases of direct ion channel gating by cyclic nucleotides. J Bioenerg Biomembr. 1991 Aug;23(4):577–597. doi: 10.1007/BF00785812. [DOI] [PubMed] [Google Scholar]
- Laurenza A., Sutkowski E. M., Seamon K. B. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci. 1989 Nov;10(11):442–447. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Matthews G. Ion channels that are directly activated by cyclic nucleotides. Trends Pharmacol Sci. 1991 Jul;12(7):245–247. doi: 10.1016/0165-6147(91)90563-8. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991 Dec;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Quast U., Baumlin Y. Comparison of the effluxes of 42K+ and 86Rb+ elicited by cromakalim (BRL 34915) in tonic and phasic vascular tissue. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):319–326. doi: 10.1007/BF00173407. [DOI] [PubMed] [Google Scholar]
- Quast U., Bray K. M., Andres H., Manley P. W., Baumlin Y., Dosogne J. Binding of the K+ channel opener [3H]P1075 in rat isolated aorta: relationship to functional effects of openers and blockers. Mol Pharmacol. 1993 Mar;43(3):474–481. [PubMed] [Google Scholar]
- Quast U., Cook N. S. In vitro and in vivo comparison of two K+ channel openers, diazoxide and cromakalim, and their inhibition by glibenclamide. J Pharmacol Exp Ther. 1989 Jul;250(1):261–271. [PubMed] [Google Scholar]
- Quast U. Do the K+ channel openers relax smooth muscle by opening K+ channels? Trends Pharmacol Sci. 1993 Sep;14(9):332–337. doi: 10.1016/0165-6147(93)90006-6. [DOI] [PubMed] [Google Scholar]
- Quast U. Effect of the K+ efflux stimulating vasodilator BRL 34915 on 86Rb+ efflux and spontaneous activity in guinea-pig portal vein. Br J Pharmacol. 1987 Jul;91(3):569–578. doi: 10.1111/j.1476-5381.1987.tb11250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. M., Bonev A. D., Brayden J. E., Nelson M. T. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994 Feb 15;475(1):9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. I., Ertel P., Smallwood J. K., Wyss V., Zimmerman K. The relation between vascular relaxant and cardiac electrophysiological effects of pinacidil. J Cardiovasc Pharmacol. 1988;12 (Suppl 2):S30–S40. doi: 10.1097/00005344-198812002-00007. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K., Wang W., Giebisch G., Welling P. A. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6418–6422. doi: 10.1073/pnas.89.14.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. H., Schwab A., Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol. 1990 Sep;259(3 Pt 2):F494–F502. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- Wang W. H., White S., Geibel J., Giebisch G. A potassium channel in the apical membrane of rabbit thick ascending limb of Henle's loop. Am J Physiol. 1990 Feb;258(2 Pt 2):F244–F253. doi: 10.1152/ajprenal.1990.258.2.F244. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Feramisco J. R., Casnellie J. E., Krebs E. G., Walsh D. A. Studies on the kinetic mechanism of the catalytic subunit of the cAMP-dependent protein kinase. J Biol Chem. 1983 Mar 25;258(6):3693–3701. [PubMed] [Google Scholar]
- de Weille J. R. Modulation of ATP sensitive potassium channels. Cardiovasc Res. 1992 Nov;26(11):1017–1020. doi: 10.1093/cvr/26.11.1017. [DOI] [PubMed] [Google Scholar]


