Abstract
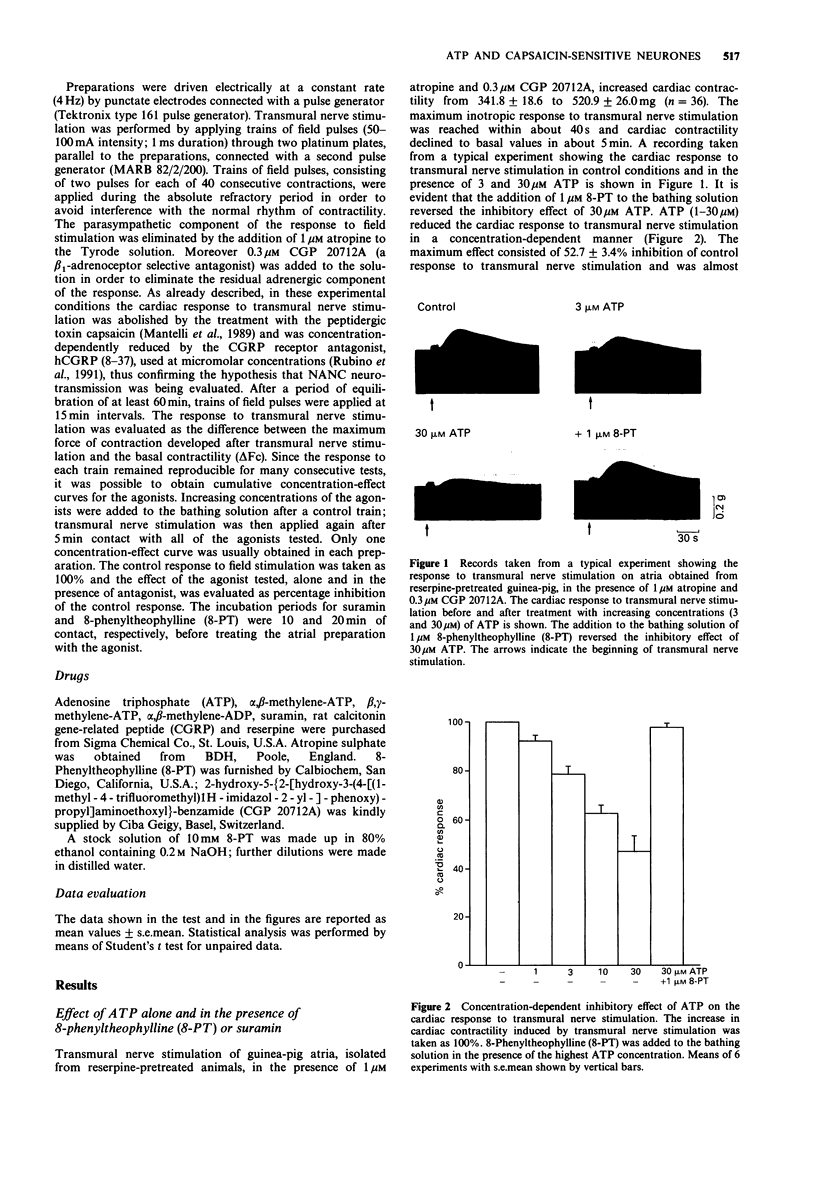
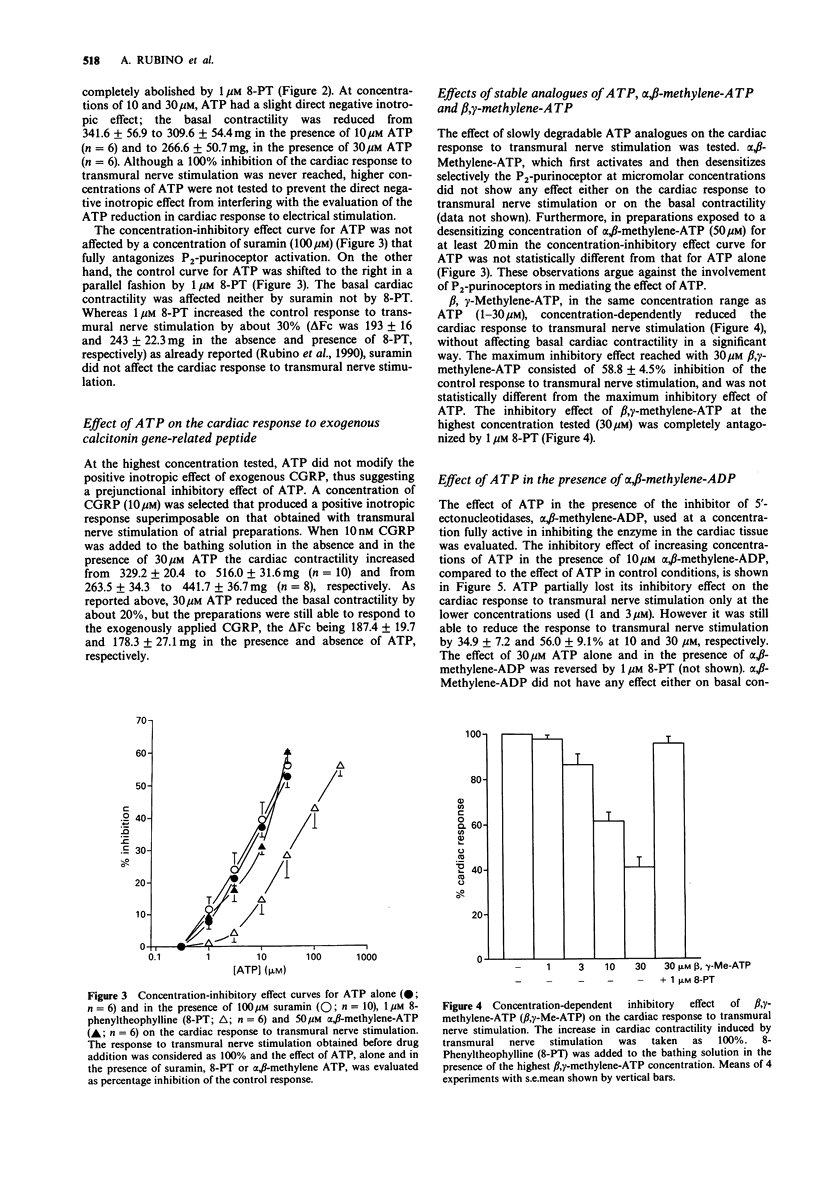
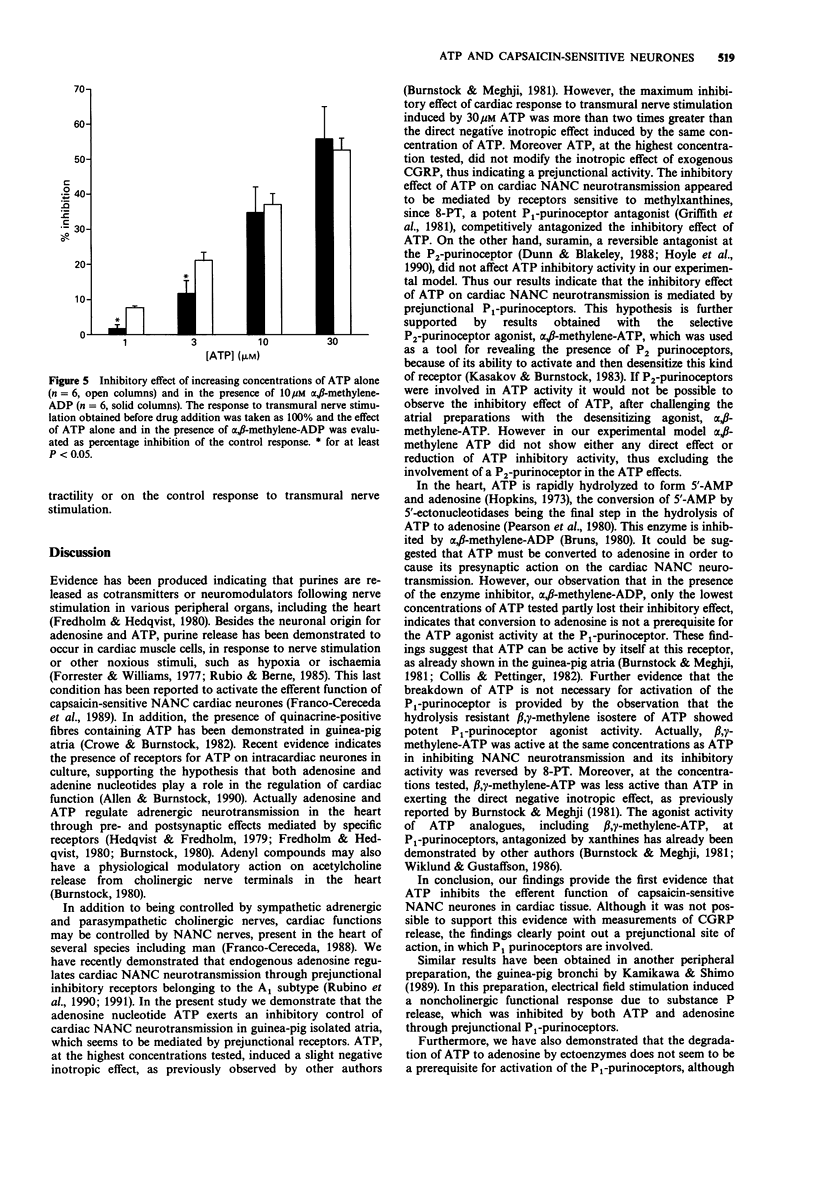
1. The effect of adenosine triphosphate (ATP) and its stable analogues, alpha, beta-methylene-ATP and beta, gamma-methylene-ATP, on the efferent function of capsaicin-sensitive non-adrenergic, non-cholinergic (NANC) nerves was tested in guinea-pig isolated atria. 2. Transmural nerve stimulation of atria isolated from reserpine-pretreated guinea-pigs, in the presence of 1 microM atropine and 0.3 microM CGP 20712A, induced a transient positive inotropic effect attributable to calcitonin gene-related peptide (CGRP) release from NANC nerve endings. 3. ATP (1-30 microM) concentration-dependently reduced the cardiac response to transmural nerve stimulation, without affecting the inotropic response to 10 nM exogenous CGRP. The inhibitory effect of ATP was competitively antagonized by the P1-purinoceptor antagonist, 8-phenyltheophylline (8-PT, 1 microM), but was unaffected by the P2-purinoceptor antagonist, suramin (100 microM). 4. beta, gamma-methylene-ATP in the same concentration range as ATP, inhibited the cardiac response to transmural nerve stimulation. The inhibitory effect of beta, gamma-methylene ATP was antagonized by 1 microM 8-PT. The desensitizing agonist for P2-purinoceptors, alpha, beta-methylene ATP did not induce any inhibitory effect either on the cardiac response to transmural nerve stimulation or on the inhibitory effect curve for ATP. 5. The inhibitory effect of ATP on the NANC neurotransmission was inconsistently modified in the presence of 10 microM alpha, beta-methylene-adenosine diphosphate, an inhibitor of the 5'-ectonucleotidases. 6. These results demonstrate that ATP modulates the efferent function of cardiac NANC nerve endings through prejunctional inhibitory receptors belonging to the P1 type.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. G., Burnstock G. The actions of adenosine 5'-triphosphate on guinea-pig intracardiac neurones in culture. Br J Pharmacol. 1990 Jun;100(2):269–276. doi: 10.1111/j.1476-5381.1990.tb15794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F. Adenosine receptor activation by adenine nucleotides requires conversion of the nucleotides to adenosine. Naunyn Schmiedebergs Arch Pharmacol. 1980;315(1):5–13. doi: 10.1007/BF00504224. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Meghji P. Distribution of P1- and P2-purinoceptors in the guinea-pig and frog heart. Br J Pharmacol. 1981 Aug;73(4):879–885. doi: 10.1111/j.1476-5381.1981.tb08741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co-transmission. Arch Int Pharmacodyn Ther. 1990 Mar-Apr;304:7–33. [PubMed] [Google Scholar]
- Collis M. G., Pettinger S. J. Can ATP stimulate P1-receptors in guinea-pig atrium without conversion to adenosine? Eur J Pharmacol. 1982 Jul 30;81(4):521–529. doi: 10.1016/0014-2999(82)90341-7. [DOI] [PubMed] [Google Scholar]
- Crowe R., Burnstock G. Fluorescent histochemical localisation of quinacrine-positive neurones in the guinea-pig and rabbit atrium. Cardiovasc Res. 1982 Jul;16(7):384–390. doi: 10.1093/cvr/16.7.384. [DOI] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Williams C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun;268(2):371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Cereceda A. Calcitonin gene-related peptide and tachykinins in relation to local sensory control of cardiac contractility and coronary vascular tone. Acta Physiol Scand Suppl. 1988;569:1–63. [PubMed] [Google Scholar]
- Franco-Cereceda A., Saria A., Lundberg J. M. Differential release of calcitonin gene-related peptide and neuropeptide Y from the isolated heart by capsaicin, ischaemia, nicotine, bradykinin and ouabain. Acta Physiol Scand. 1989 Feb;135(2):173–187. doi: 10.1111/j.1748-1716.1989.tb08565.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15;29(12):1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- Giuliani S., Amann R., Papini A. M., Maggi C. A., Meli A. Modulatory action of galanin on responses due to antidromic activation of peripheral terminals of capsaicin-sensitive sensory nerves. Eur J Pharmacol. 1989 Apr 12;163(1):91–96. doi: 10.1016/0014-2999(89)90399-3. [DOI] [PubMed] [Google Scholar]
- Giuliani S., Maggi C. A., Meli A. Opioid receptors and prejunctional modulation of capsaicin-sensitive sensory nerves in guinea-pig left atrium. Gen Pharmacol. 1990;21(4):417–421. doi: 10.1016/0306-3623(90)90691-e. [DOI] [PubMed] [Google Scholar]
- Giuliani S., Maggi C. A., Meli A. Prejunctional modulatory action of neuropeptide Y on peripheral terminals of capsaicin-sensitive sensory nerves. Br J Pharmacol. 1989 Oct;98(2):407–412. doi: 10.1111/j.1476-5381.1989.tb12611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S. G., Meghji P., Moody C. J., Burnstock G. 8-phenyltheophylline: a potent P1-purinoceptor antagonist. Eur J Pharmacol. 1981 Oct 15;75(1):61–64. doi: 10.1016/0014-2999(81)90346-0. [DOI] [PubMed] [Google Scholar]
- Hedqvist P., Fredholm B. B. Inhibitory effect of adenosine on adrenergic neuroeffector transmission in the rabbit heart. Acta Physiol Scand. 1979 Jan;105(1):120–122. doi: 10.1111/j.1748-1716.1979.tb06321.x. [DOI] [PubMed] [Google Scholar]
- Hopkins S. V. The action of ATP in the guinea-pig heart. Biochem Pharmacol. 1973 Feb 1;22(3):335–339. doi: 10.1016/0006-2952(73)90414-0. [DOI] [PubMed] [Google Scholar]
- Hougland M. W., Durkee K. H., Hougland A. E. Innervation of guinea pig heart by neurons sensitive to capsaicin. J Auton Nerv Syst. 1986 Mar;15(3):217–225. doi: 10.1016/0165-1838(86)90065-2. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikawa Y., Shimo Y. Adenosine selectively inhibits noncholinergic transmission in guinea pig bronchi. J Appl Physiol (1985) 1989 May;66(5):2084–2091. doi: 10.1152/jappl.1989.66.5.2084. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Chiba T., Giuliani S. Human alpha-calcitonin gene-related peptide-(8-37) as an antagonist of exogenous and endogenous calcitonin gene-related peptide. Eur J Pharmacol. 1991 Jan 3;192(1):85–88. doi: 10.1016/0014-2999(91)90072-x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Mantelli L., Amerini S., Ledda F. Effects of opioid drugs on capsaicin-sensitive neurones in guinea-pig atria. Eur J Pharmacol. 1989 Nov 7;170(3):217–223. doi: 10.1016/0014-2999(89)90542-6. [DOI] [PubMed] [Google Scholar]
- Mantelli L., Amerini S., Rubino A., Ledda F. Characterization of opioid receptors modulating the function of capsaicin-sensitive neurons in guinea-pig atria. Eur J Pharmacol. 1990 May 16;180(2-3):325–330. doi: 10.1016/0014-2999(90)90317-y. [DOI] [PubMed] [Google Scholar]
- Miyauchi T., Ishikawa T., Sugishita Y., Saito A., Goto K. Effects of capsaicin on nonadrenergic noncholinergic nerves in the guinea pig atria: role of calcitonin gene-related peptide as cardiac neurotransmitter. J Cardiovasc Pharmacol. 1987 Dec;10(6):675–682. doi: 10.1097/00005344-198712000-00011. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Gordon J. L. Metabolism of adenine nucleotides by ectoenzymes of vascular endothelial and smooth-muscle cells in culture. Biochem J. 1980 Aug 15;190(2):421–429. doi: 10.1042/bj1900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino A., Amerini S., Mantelli L., Ledda F. Adenosine receptors involved in the inhibitory control of non-adrenergic non-cholinergic neurotransmission in guinea-pig atria belong to the A1 subtype. Naunyn Schmiedebergs Arch Pharmacol. 1991 Oct;344(4):464–470. doi: 10.1007/BF00172587. [DOI] [PubMed] [Google Scholar]
- Rubino A., Mantelli L., Amerini S., Ledda F. Adenosine modulation of non-adrenergic non-cholinergic neurotransmission in isolated guinea-pig atria. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):520–522. doi: 10.1007/BF00169039. [DOI] [PubMed] [Google Scholar]
- Saito A., Ishikawa T., Kimura S., Goto K. Role of calcitonin gene-related peptide as cardiotonic neurotransmitter in guinea pig left atria. J Pharmacol Exp Ther. 1987 Nov;243(2):731–736. [PubMed] [Google Scholar]
- Saito A., Ishikawa T., Masaki T., Kimura S., Goto K. Pharmacological analysis of autonomic innervation of the right atria of rats and guinea pigs: demonstration of nonadrenergic noncholinergic nerves. J Pharmacol Exp Ther. 1986 Aug;238(2):713–719. [PubMed] [Google Scholar]
- Wharton J., Gulbenkian S., Mulderry P. K., Ghatei M. A., McGregor G. P., Bloom S. R., Polak J. M. Capsaicin induces a depletion of calcitonin gene-related peptide (CGRP)-immunoreactive nerves in the cardiovascular system of the guinea pig and rat. J Auton Nerv Syst. 1986 Aug;16(4):289–309. doi: 10.1016/0165-1838(86)90035-4. [DOI] [PubMed] [Google Scholar]
- Wiklund N. P., Gustafsson L. E. Neuromodulation by adenine nucleotides, as indicated by experiments with inhibitors of nucleotide inactivation. Acta Physiol Scand. 1986 Feb;126(2):217–223. doi: 10.1111/j.1748-1716.1986.tb07808.x. [DOI] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]


