Abstract
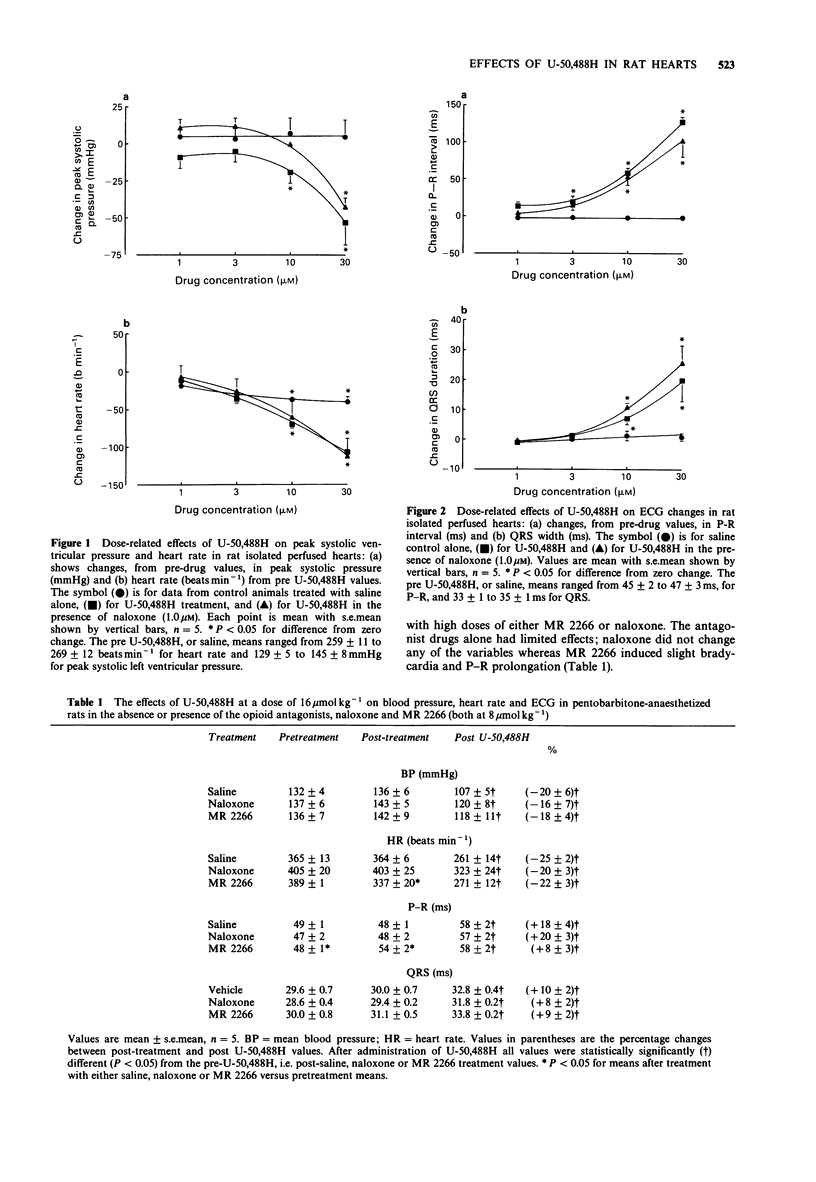
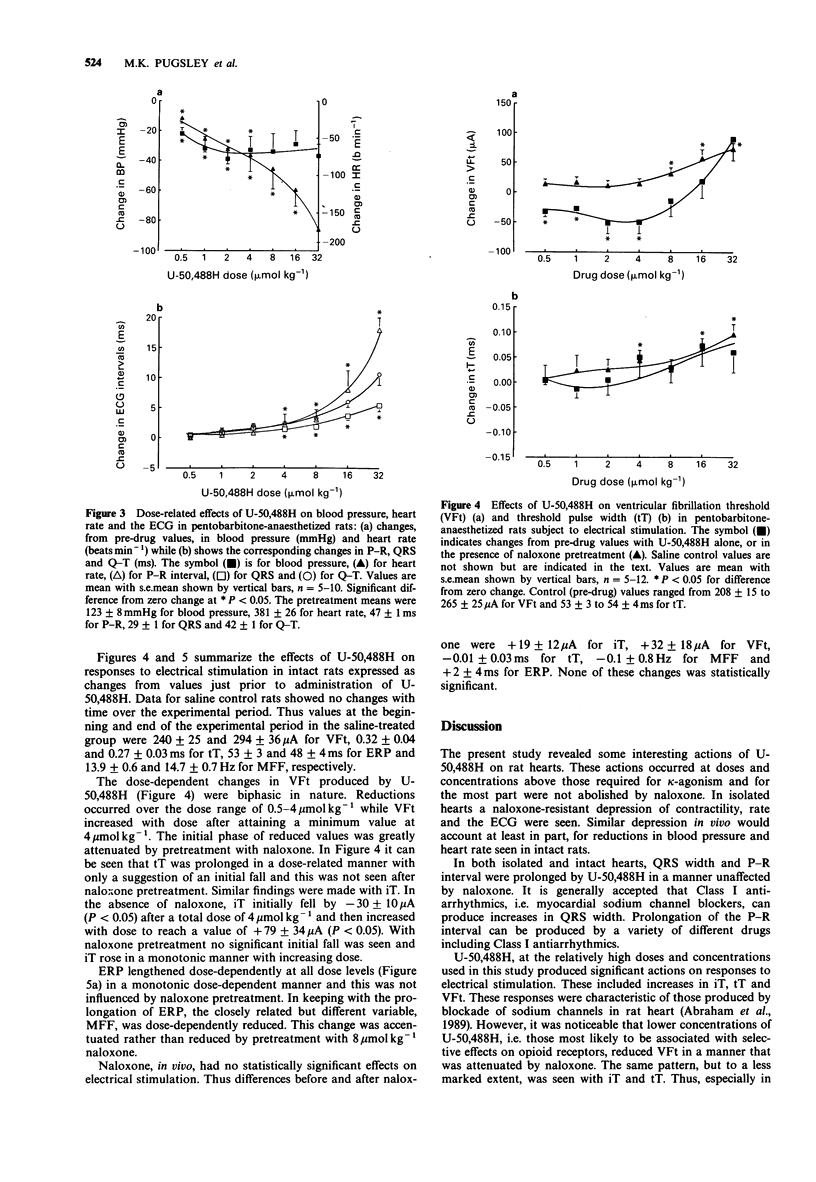
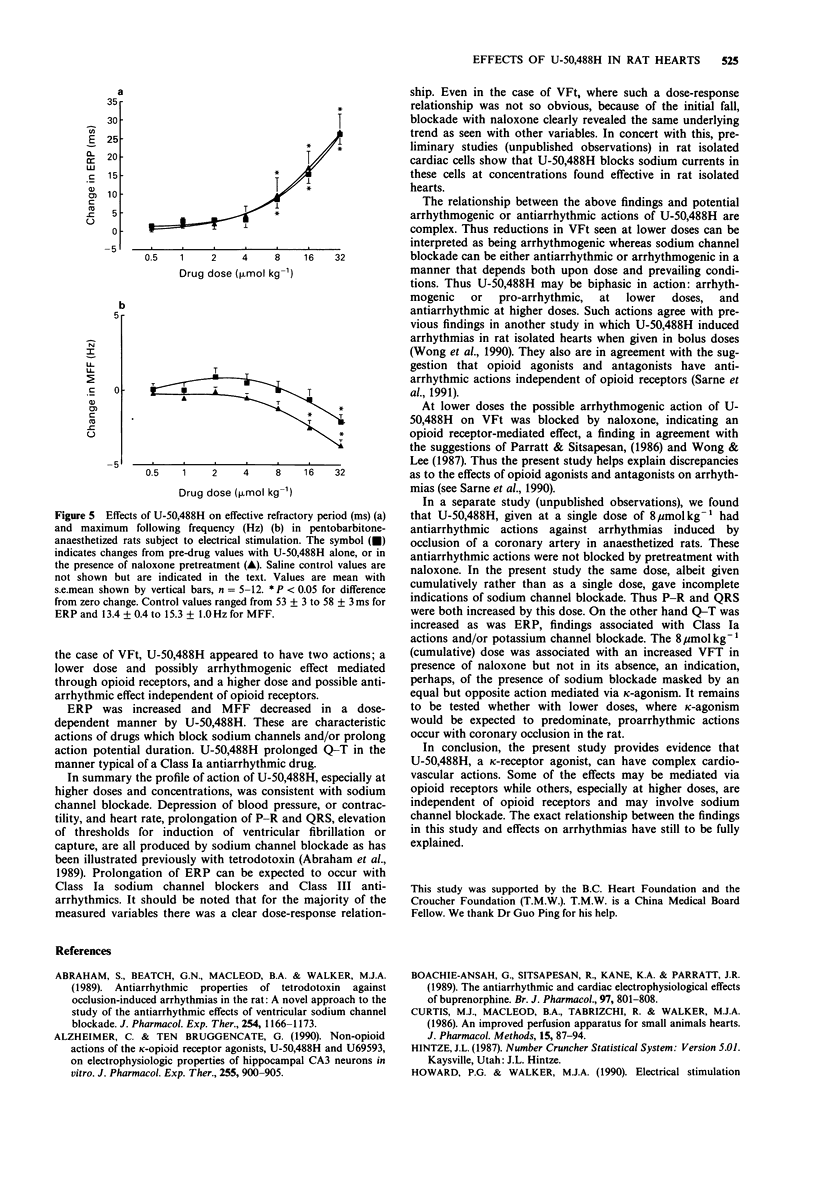
1. The cardiovascular actions of U-50,488H, a kappa-receptor agonist, were studied in rat isolated perfused hearts, and in anaesthetized rats, over concentrations or doses generally above those required to produce kappa-receptor-mediated effects. 2. U-50,488H dose-dependently decreased left-ventricular peak systolic pressure and beating rate in vitro and reduced blood pressure and heart rate in vivo. 3. Over the concentration range of 1-30 microM in vitro, and the dose-range of 0.5-32 mumol kg-1 in vivo, U-50,488H prolonged the P-R, QRS and Q-T intervals of the ECG. 4. The effects of U-50,488H were not antagonized by an opioid receptor antagonist, naloxone (1 microM or 8 mumol kg-1). Similarly, the opioid receptor antagonist, MR 2266, at 8 mumol kg-1 did not significantly reduce the cardiovascular actions of U-50,488H in vivo. 5. The actions of U-50,488H on responses to electrical stimulation were also studied. Over the dose range of 0.5-32 mumol kg-1, U-50,488H altered thresholds and effective refractory period. It had a biphasic action on thresholds for induction of ventricular fibrillation. Thresholds were decreased at lower doses (0.5-4 mumol kg-1) but increased at higher doses (8-32 mumol kg-1). The effects of lower doses were blocked by naloxone. Effective refractory period and threshold pulse width only increased with dose. 6. In conclusion, U-50,488H at high concentration, had direct depressant actions on cardiac contractility, electrical excitability and the ECG. These depressant effects were not antagonized by the opioid receptor antagonists, naloxone and MR 2266, and probably do not involve opioid receptors.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S., Beatch G. N., MacLeod B. A., Walker M. J. Antiarrhythmic properties of tetrodotoxin against occlusion-induced arrhythmias in the rat: a novel approach to the study of the antiarrhythmic effects of ventricular sodium channel blockade. J Pharmacol Exp Ther. 1989 Dec;251(3):1166–1173. [PubMed] [Google Scholar]
- Alzheimer C., ten Bruggencate G. Nonopioid actions of the kappa-opioid receptor agonists, U 50488H and U 69593 on electrophysiologic properties of hippocampal CA3 neurons in vitro. J Pharmacol Exp Ther. 1990 Nov;255(2):900–905. [PubMed] [Google Scholar]
- Boachie-Ansah G., Sitsapesan R., Kane K. A., Parratt J. R. The antiarrhythmic and cardiac electrophysiological effects of buprenorphine. Br J Pharmacol. 1989 Jul;97(3):801–808. doi: 10.1111/j.1476-5381.1989.tb12019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M. J., Macleod B. A., Tabrizchi R., Walker M. J. An improved perfusion apparatus for small animal hearts. J Pharmacol Methods. 1986 Feb;15(1):87–94. doi: 10.1016/0160-5402(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Howard P. G., Walker M. J. Electrical stimulation studies with quinacainol, a putative 1C agent, in the anaesthetised rat. Proc West Pharmacol Soc. 1990;33:123–127. [PubMed] [Google Scholar]
- Lahti R. A., VonVoigtlander P. F., Barsuhn C. Properties of a selective kappa agonist, U-50,488H. Life Sci. 1982 Nov 15;31(20-21):2257–2260. doi: 10.1016/0024-3205(82)90132-1. [DOI] [PubMed] [Google Scholar]
- Martin W. R. Pharmacology of opioids. Pharmacol Rev. 1983 Dec;35(4):283–323. [PubMed] [Google Scholar]
- Parratt J. R., Sitsapesan R. Stereospecific antiarrhythmic effect of opioid receptor antagonists in myocardial ischaemia. Br J Pharmacol. 1986 Apr;87(4):621–622. doi: 10.1111/j.1476-5381.1986.tb14577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarne Y., Flitstein A., Oppenheimer E. Anti-arrhythmic activities of opioid agonists and antagonists and their stereoisomers. Br J Pharmacol. 1991 Mar;102(3):696–698. doi: 10.1111/j.1476-5381.1991.tb12235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Parratt J. R. The effects of drugs interacting with opioid receptors on the early ventricular arrhythmias arising from myocardial ischaemia. Br J Pharmacol. 1989 Jul;97(3):795–800. doi: 10.1111/j.1476-5381.1989.tb12018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. J., Beatch G. N. Electrically induced arrhythmias in the rat. Proc West Pharmacol Soc. 1988;31:167–170. [PubMed] [Google Scholar]
- Wong T. M., Lee A. Y. Chronic morphine treatment reduces the incidence of ventricular arrhythmias in the isolated rat heart induced by dynorphin1-13 or myocardial ischemia and reperfusion. Neurosci Lett. 1987 Jun 1;77(1):61–65. doi: 10.1016/0304-3940(87)90607-0. [DOI] [PubMed] [Google Scholar]
- Wong T. M., Lee A. Y., Tai K. K. Effects of drugs interacting with opioid receptors during normal perfusion or ischemia and reperfusion in the isolated rat heart--an attempt to identify cardiac opioid receptor subtype(s) involved in arrhythmogenesis. J Mol Cell Cardiol. 1990 Oct;22(10):1167–1175. doi: 10.1016/0022-2828(90)90080-l. [DOI] [PubMed] [Google Scholar]


