Abstract
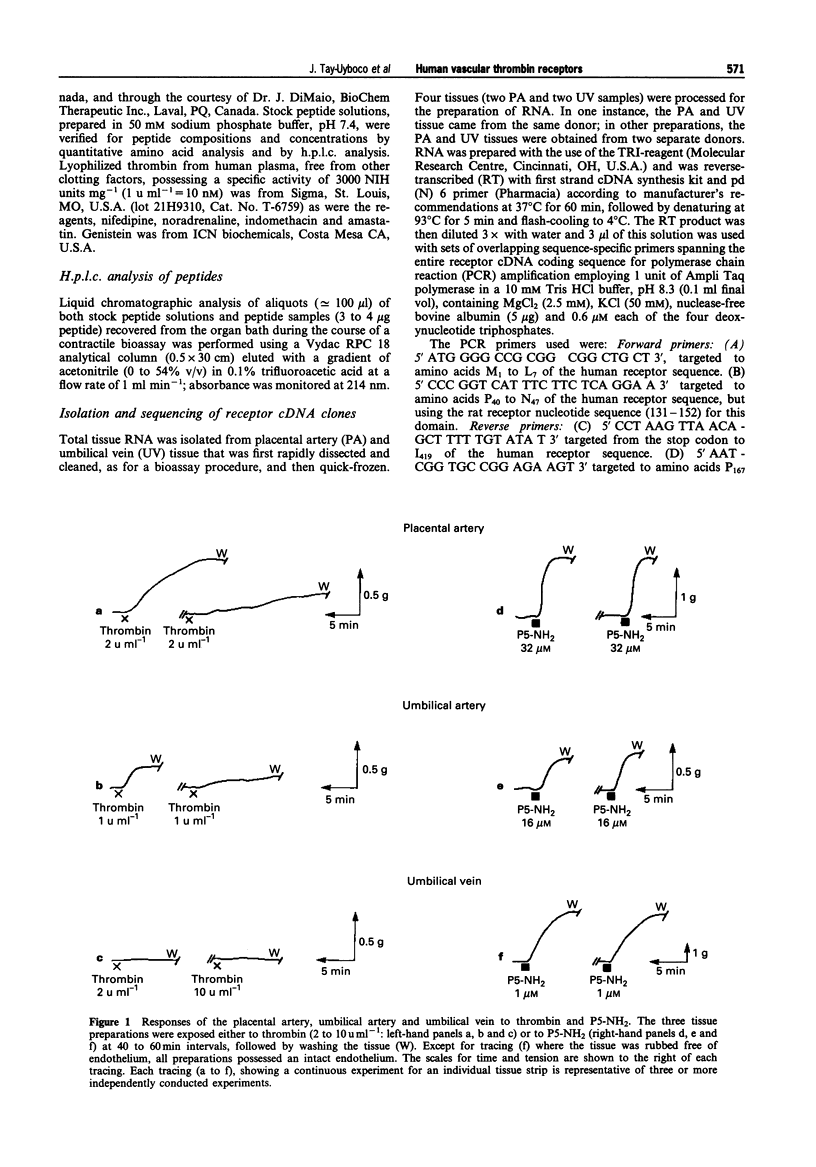
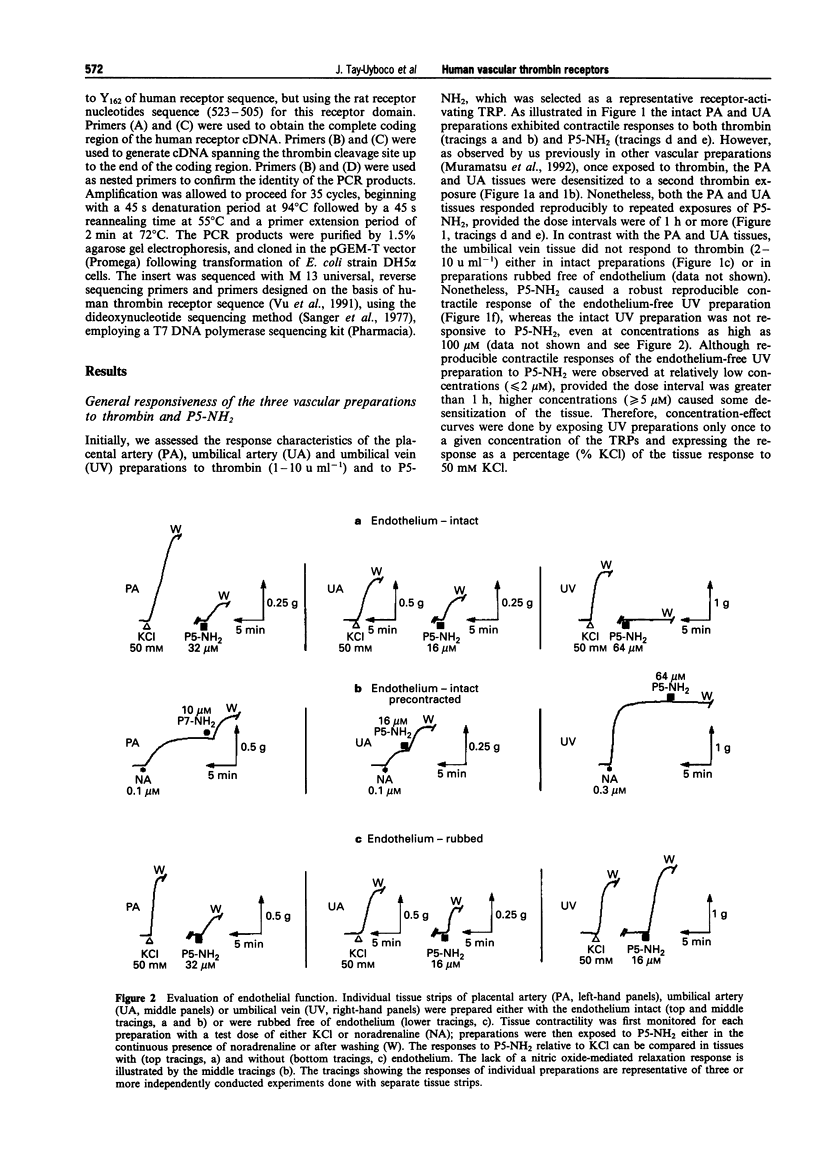
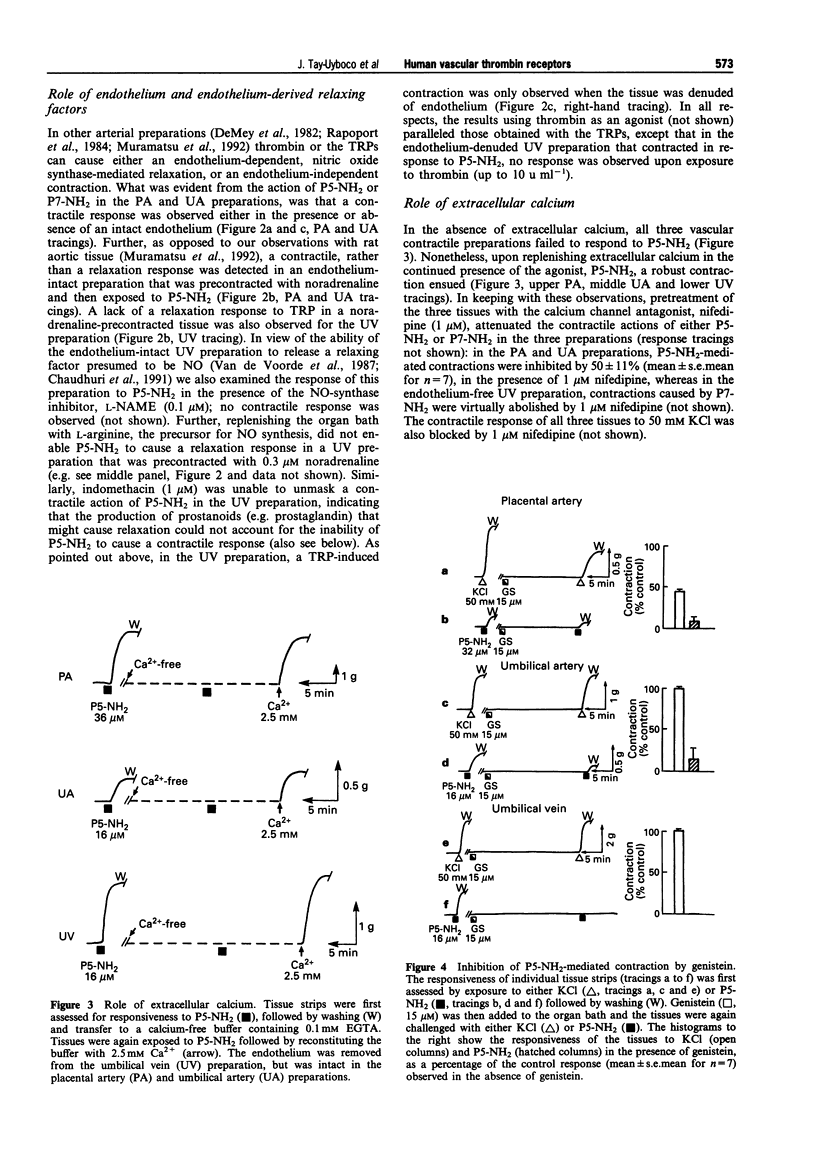
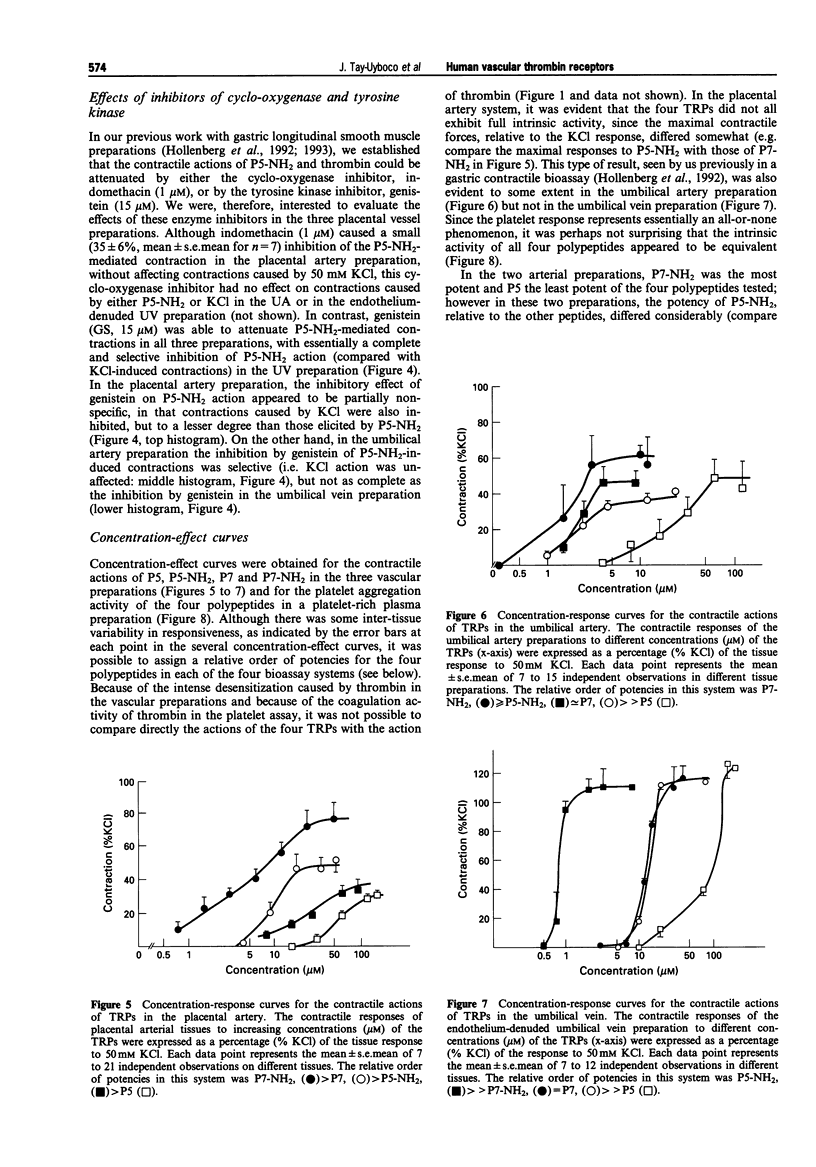
1. We studied the structure-activity profiles of four thrombin receptor-derived polypeptides (TRPs) (P5, SFLLR; P5-NH2, SFLLR-NH2; P7, SFLLRNP; P7-NH2, SFLLRN) in contractile human placental artery (PA), umbilical artery (UA) and umbilical vein (UV) preparations and in a human platelet aggregation assay. 2. The contractile actions of the TRPs in the two arterial preparations were endothelium-independent, whereas in the UV tissue a contractile response was observed only in an endothelium-denuded preparation; no endothelium-mediated relaxation responses were observed in any of the vascular preparations. 3. In the three vascular preparations, the contractile responses required extracellular calcium and were attenuated by the tyrosine kinase inhibitor, genistein. 4. The relative contractile orders of potencies of the TRPs in the three vascular preparations were distinct from each other (PA: P7-NH2 > P7 > P5-NH2 > P5; UA: P7-NH2 > or = P5-NH2 approximately = P7 > > P5; UV: P5-NH2 > > P7-NH2 = P7 > > P5) and these were in turn distinct from the potency order observed in the platelet aggregation assay (P5-NH2 > or = P7-NH2 > P7 > > P5). 5. Despite the markedly dissimilar TRP potency orders in the placental artery and umbilical vein preparations, the cDNA sequences for the thrombin receptor obtained by polymerase chain reaction cloning of cDNA from the two tissue sources were identical. 6. We conclude that the four tissues studied possess functionally distinct thrombin receptor systems that interact in a distinct way with agonist peptides. In view of the identity of the thrombin receptor cDNA in the two tissues displaying the most dissimilar structure-activity profiles, we suggest that in different tissues, differences in post-translational receptor processing or differences in receptor-effector coupling interactions may result in unique thrombin receptor systems that can display distinct structure-activity profiles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonaccio M. J., Normandin D., Serafino R., Moreland S. Effects of thrombin and thrombin receptor activating peptides on rat aortic vascular smooth muscle. J Pharmacol Exp Ther. 1993 Jul;266(1):125–132. [PubMed] [Google Scholar]
- Bahou W. F., Coller B. S., Potter C. L., Norton K. J., Kutok J. L., Goligorsky M. S. The thrombin receptor extracellular domain contains sites crucial for peptide ligand-induced activation. J Clin Invest. 1993 Apr;91(4):1405–1413. doi: 10.1172/JCI116344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Buttery L. D., McCarthy A., Springall D. R., Sullivan M. H., Elder M. G., Michel T., Polak J. M. Endothelial nitric oxide synthase in the human placenta: regional distribution and proposed regulatory role at the feto-maternal interface. Placenta. 1994 Apr;15(3):257–265. doi: 10.1016/0143-4004(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Chao B. H., Kalkunte S., Maraganore J. M., Stone S. R. Essential groups in synthetic agonist peptides for activation of the platelet thrombin receptor. Biochemistry. 1992 Jul 14;31(27):6175–6178. doi: 10.1021/bi00142a001. [DOI] [PubMed] [Google Scholar]
- Chaudhuri G., Buga G. M., Gold M. E., Wood K. S., Ignarro L. J. Characterization and actions of human umbilical endothelium derived relaxing factor. Br J Pharmacol. 1991 Feb;102(2):331–336. doi: 10.1111/j.1476-5381.1991.tb12174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Ward P., Ceruso M., Scudder L. E., Springer K., Kutok J., Prestwich G. D. Thrombin receptor activating peptides: importance of the N-terminal serine and its ionization state as judged by pH dependence, nuclear magnetic resonance spectroscopy, and cleavage by aminopeptidase M. Biochemistry. 1992 Dec 1;31(47):11713–11720. doi: 10.1021/bi00162a007. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Vu T. K., Hung D. T., Wheaton V. I. Characterization of a functional thrombin receptor. Issues and opportunities. J Clin Invest. 1992 Feb;89(2):351–355. doi: 10.1172/JCI115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- Gerszten R. E., Chen J., Ishii M., Ishii K., Wang L., Nanevicz T., Turck C. W., Vu T. K., Coughlin S. R. Specificity of the thrombin receptor for agonist peptide is defined by its extracellular surface. Nature. 1994 Apr 14;368(6472):648–651. doi: 10.1038/368648a0. [DOI] [PubMed] [Google Scholar]
- Godin D., Marceau F., Beaulé C., Rioux F., Drapeau G. Aminopeptidase modulation of the pharmacological responses to synthetic thrombin receptor agonists. Eur J Pharmacol. 1994 Mar 3;253(3):225–230. doi: 10.1016/0014-2999(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Haver V. M., Namm D. H. Characterization of the thrombin-induced contraction of vascular smooth muscle. Blood Vessels. 1984;21(2):53–63. [PubMed] [Google Scholar]
- Hollenberg M. D., Laniyonu A. A., Saifeddine M., Moore G. J. Role of the amino- and carboxyl-terminal domains of thrombin receptor-derived polypeptides in biological activity in vascular endothelium and gastric smooth muscle: evidence for receptor subtypes. Mol Pharmacol. 1993 Jun;43(6):921–930. [PubMed] [Google Scholar]
- Hollenberg M. D., Yang S. G., Laniyonu A. A., Moore G. J., Saifeddine M. Action of thrombin receptor polypeptide in gastric smooth muscle: identification of a core pentapeptide retaining full thrombin-mimetic intrinsic activity. Mol Pharmacol. 1992 Aug;42(2):186–191. [PubMed] [Google Scholar]
- Hui K. Y., Jakubowski J. A., Wyss V. L., Angleton E. L. Minimal sequence requirement of thrombin receptor agonist peptide. Biochem Biophys Res Commun. 1992 Apr 30;184(2):790–796. doi: 10.1016/0006-291x(92)90659-9. [DOI] [PubMed] [Google Scholar]
- Hung D. T., Wong Y. H., Vu T. K., Coughlin S. R. The cloned platelet thrombin receptor couples to at least two distinct effectors to stimulate phosphoinositide hydrolysis and inhibit adenylyl cyclase. J Biol Chem. 1992 Oct 15;267(29):20831–20834. [PubMed] [Google Scholar]
- Kenakin T. P., Morgan P. H. Theoretical effects of single and multiple transducer receptor coupling proteins on estimates of the relative potency of agonists. Mol Pharmacol. 1989 Feb;35(2):214–222. [PubMed] [Google Scholar]
- McIntyre P., Phillips E., Skidmore E., Brown M., Webb M. Cloned murine bradykinin receptor exhibits a mixed B1 and B2 pharmacological selectivity. Mol Pharmacol. 1993 Aug;44(2):346–355. [PubMed] [Google Scholar]
- Muramatsu I., Laniyonu A., Moore G. J., Hollenberg M. D. Vascular actions of thrombin receptor peptide. Can J Physiol Pharmacol. 1992 Jul;70(7):996–1003. doi: 10.1139/y92-137. [DOI] [PubMed] [Google Scholar]
- Nelken N. A., Soifer S. J., O'Keefe J., Vu T. K., Charo I. F., Coughlin S. R. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992 Oct;90(4):1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt S., Emilsson K., Wahlestedt C., Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Mechanisms of adenosine triphosphate-, thrombin-, and trypsin-induced relaxation of rat thoracic aorta. Circ Res. 1984 Oct;55(4):468–479. doi: 10.1161/01.res.55.4.468. [DOI] [PubMed] [Google Scholar]
- Rasmussen U. B., Vouret-Craviari V., Jallat S., Schlesinger Y., Pagès G., Pavirani A., Lecocq J. P., Pouysségur J., Van Obberghen-Schilling E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991 Aug 19;288(1-2):123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- Sabo T., Gurwitz D., Motola L., Brodt P., Barak R., Elhanaty E. Structure-activity studies of the thrombin receptor activating peptide. Biochem Biophys Res Commun. 1992 Oct 30;188(2):604–610. doi: 10.1016/0006-291x(92)91099-c. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Voorde J., Vanderstichele H., Leusen I. Release of endothelium-derived relaxing factor from human umbilical vessels. Circ Res. 1987 Apr;60(4):517–522. doi: 10.1161/01.res.60.4.517. [DOI] [PubMed] [Google Scholar]
- Vassallo R. R., Jr, Kieber-Emmons T., Cichowski K., Brass L. F. Structure-function relationships in the activation of platelet thrombin receptors by receptor-derived peptides. J Biol Chem. 1992 Mar 25;267(9):6081–6085. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Walz D. A., Anderson G. F., Ciaglowski R. E., Aiken M., Fenton J. W., 2nd Thrombin-elicited contractile responses of aortic smooth muscle. Proc Soc Exp Biol Med. 1985 Dec;180(3):518–526. doi: 10.3181/00379727-180-42211. [DOI] [PubMed] [Google Scholar]
- Walz D. A., Anderson G. F., Fenton J. W., 2nd Responses of aortic smooth muscle to thrombin and thrombin analogues. Ann N Y Acad Sci. 1986;485:323–334. doi: 10.1111/j.1749-6632.1986.tb34594.x. [DOI] [PubMed] [Google Scholar]
- White R. P., Chapleau C. E., Dugdale M., Robertson J. T. Cerebral arterial contractions induced by human and bovine thrombin. Stroke. 1980 Jul-Aug;11(4):363–368. doi: 10.1161/01.str.11.4.363. [DOI] [PubMed] [Google Scholar]
- White R. P., Shirasawa Y., Robertson J. T. Comparison of responses elicited by alpha-thrombin in isolated canine basilar, coronary, mesenteric, and renal arteries. Blood Vessels. 1984;21(1):12–22. doi: 10.1159/000158490. [DOI] [PubMed] [Google Scholar]
- Xie H., Triggle C. R. Endothelium-independent relaxations to acetylcholine and A23187 in the human umbilical artery. J Vasc Res. 1994 Mar-Apr;31(2):92–105. doi: 10.1159/000159035. [DOI] [PubMed] [Google Scholar]
- Yang S. G., Laniyonu A., Saifeddine M., Moore G. J., Hollenberg M. D. Actions of thrombin and thrombin receptor peptide analogues in gastric and aortic smooth muscle: development of bioassays for structure-activity studies. Life Sci. 1992;51(17):1325–1332. doi: 10.1016/0024-3205(92)90631-x. [DOI] [PubMed] [Google Scholar]
- Yang Z., Arnet U., Bauer E., von Segesser L., Siebenmann R., Turina M., Lüscher T. F. Thrombin-induced endothelium-dependent inhibition and direct activation of platelet-vessel wall interaction. Role of prostacyclin, nitric oxide, and thromboxane A2. Circulation. 1994 May;89(5):2266–2272. doi: 10.1161/01.cir.89.5.2266. [DOI] [PubMed] [Google Scholar]
- Zhong C., Hayzer D. J., Corson M. A., Runge M. S. Molecular cloning of the rat vascular smooth muscle thrombin receptor. Evidence for in vitro regulation by basic fibroblast growth factor. J Biol Chem. 1992 Aug 25;267(24):16975–16979. [PubMed] [Google Scholar]
- deBlois D., Drapeau G., Petitclerc E., Marceau F. Synergism between the contractile effect of epidermal growth factor and that of des-Arg9-bradykinin or of alpha-thrombin in rabbit aortic rings. Br J Pharmacol. 1992 Apr;105(4):959–967. doi: 10.1111/j.1476-5381.1992.tb09085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


