Abstract
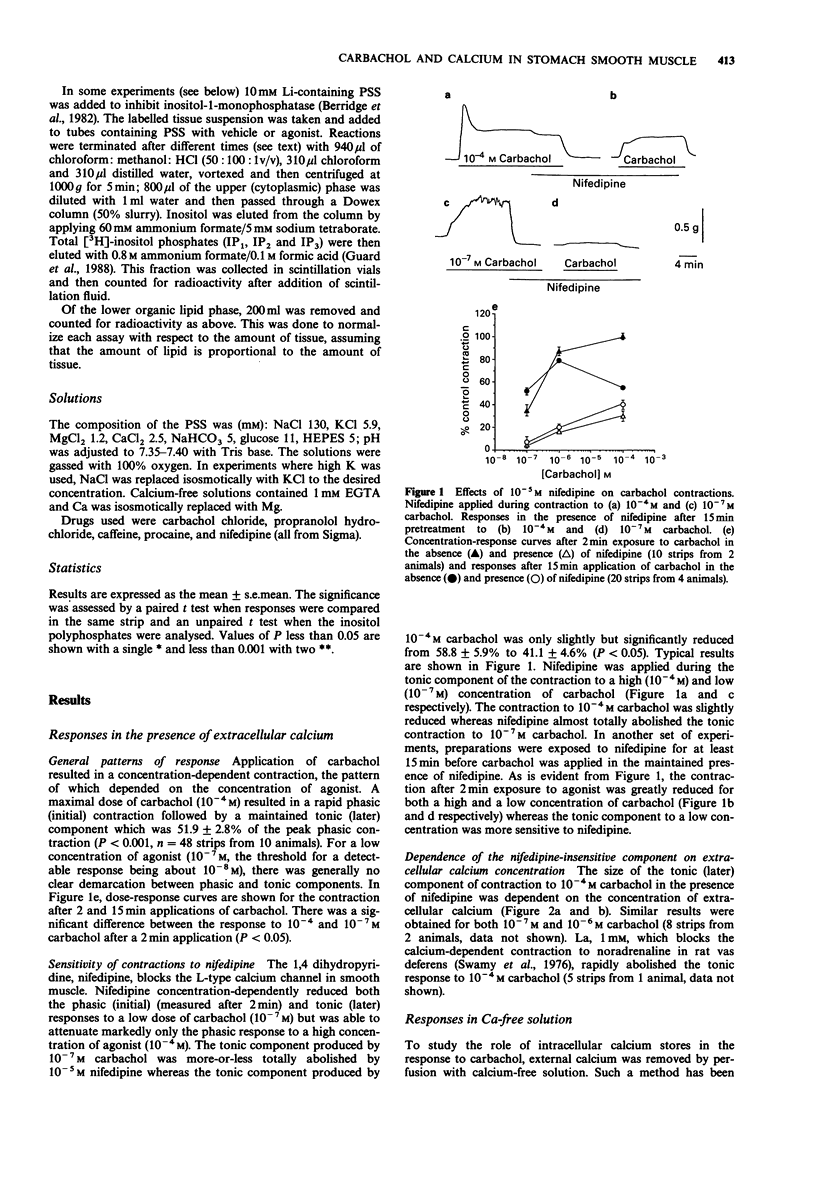
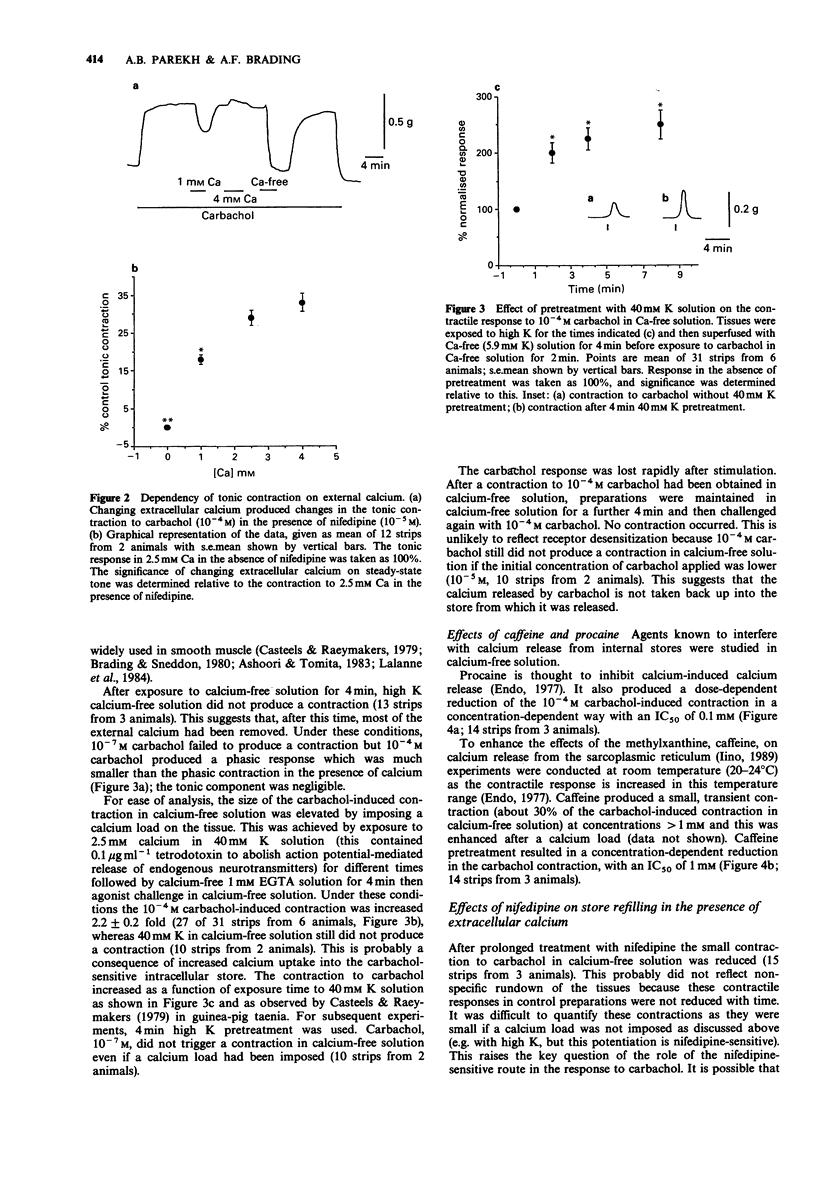
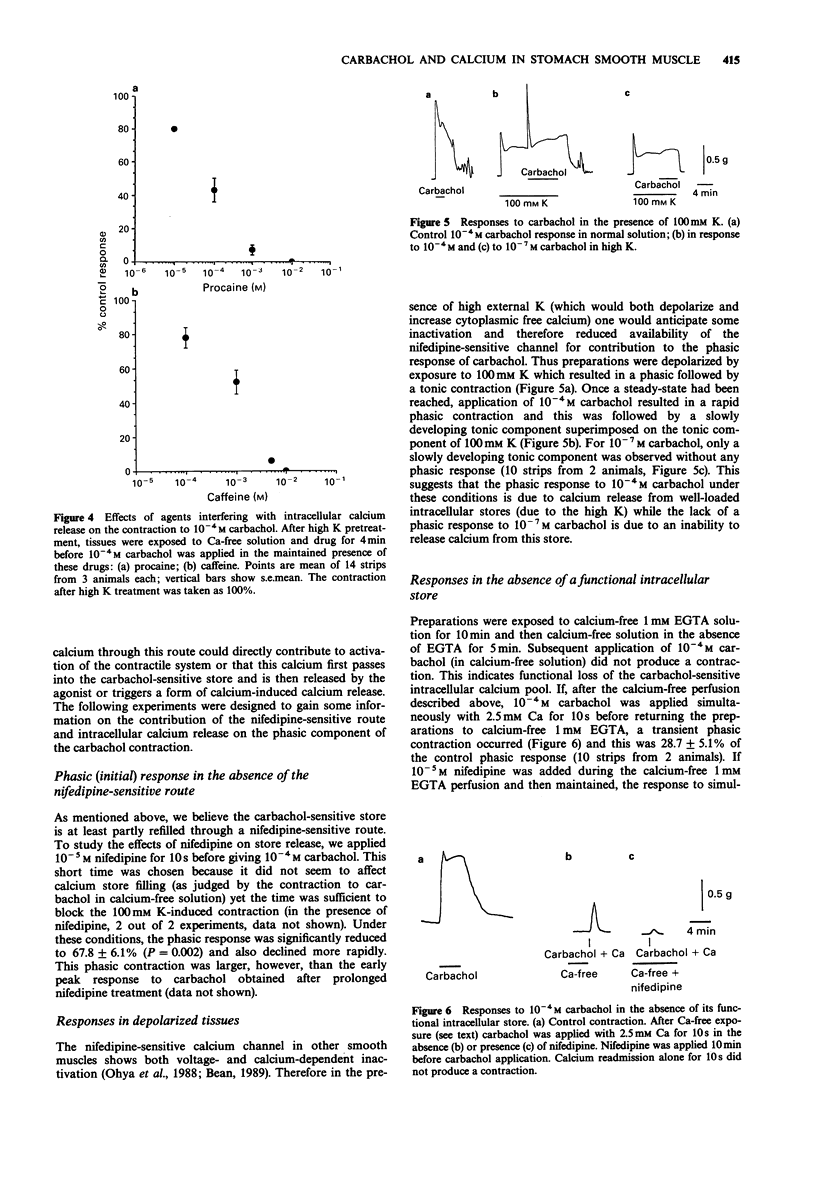
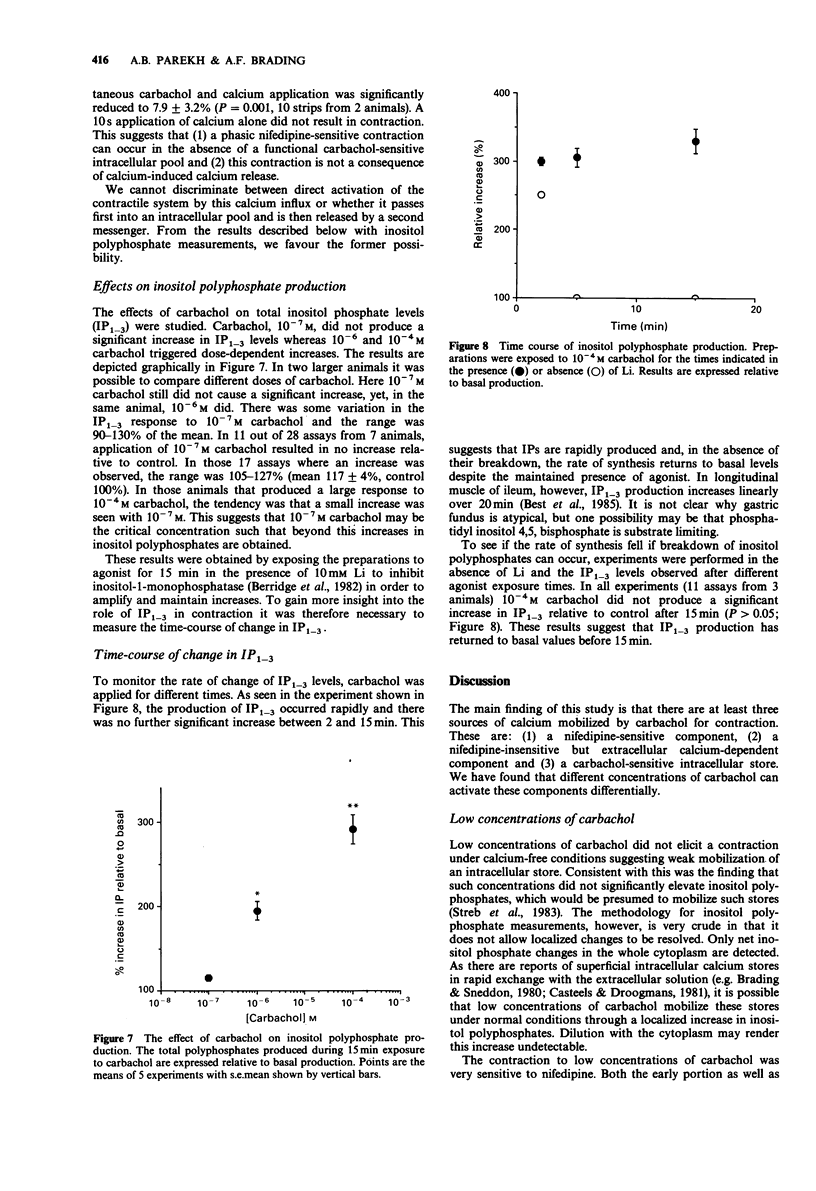
1. The action of carbachol on the mechanical activity of circular muscle from guinea-pig upper stomach was studied. High concentrations of carbachol (e.g. 10(-4) M) produced a rapid phasic contraction followed by a smaller, sustained tonic contraction. Low concentrations (e.g. 10(-7) M) caused a contraction which did not generally show marked distinction between phasic and tonic components. 2. The response to 10(-7) M carbachol was very sensitive to 10(-5) M nifedipine as was the phasic response to 10(-4) M carbachol. The tonic contraction to the latter, however, was only slightly reduced by nifedipine. 3. The carbachol-induced contractions remaining in the presence of nifedipine were dose-related and very dependent on the presence of external calcium. 4. Carbachol, 10(-7) M, did not produce a contraction after 4 min exposure to calcium-free solution whereas 10(-4) M carbachol did and this was phasic in nature but much reduced relative to the control in normal Ca. 5. A phasic followed by a small tonic contraction to 10(-4) M carbachol was seen superimposed on the K contracture in tissues depolarized with 100 mM K, whereas only a small tonic response occurred for 10(-7) M carbachol. 6. In the absence of a functional carbachol-sensitive intracellular store, 10(-4) M carbachol was unable to trigger a contraction in calcium-free solution. However, when calcium was simultaneously readmitted with carbachol after exposure to calcium-free solution, a contraction occurred. 7. Carbachol, 10(-7) M, did not significantly increase inositol polyphosphate levels, whereas 10(-4) M carbachol did.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Ashoori F., Tomita T. Mechanical response to noradrenaline in calcium-free solution in the rat vas deferens. J Physiol. 1983 May;338:165–178. doi: 10.1113/jphysiol.1983.sp014667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best L., Brooks K. J., Bolton T. B. Relationship between stimulated inositol lipid hydrolysis and contractility in guinea-pig visceral longitudinal smooth muscle. Biochem Pharmacol. 1985 Jul 1;34(13):2297–2301. doi: 10.1016/0006-2952(85)90785-3. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M. A., Fraser C. M. Muscarinic acetylcholine receptor subtypes which selectively couple to phospholipase C: pharmacological and biochemical properties. Biochem Biophys Res Commun. 1990 Dec 14;173(2):666–672. doi: 10.1016/s0006-291x(05)80087-7. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauvin C., Saida K., van Breemen C. Extracellular Ca2+ dependence and diltiazem inhibition of contraction in rabbit conduit arteries and mesenteric resistance vessels. Blood Vessels. 1984;21(1):23–31. doi: 10.1159/000158491. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Kanterman R. Y., Ma A. L., Axelrod J. A transfected m1 muscarinic acetylcholine receptor stimulates adenylate cyclase via phosphatidylinositol hydrolysis. J Biol Chem. 1989 Dec 5;264(34):20356–20362. [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard S., Watling K. J., Watson S. P. Neurokinin3-receptors are linked to inositol phospholipid hydrolysis in the guinea-pig ileum longitudinal muscle-myenteric plexus preparation. Br J Pharmacol. 1988 May;94(1):148–154. doi: 10.1111/j.1476-5381.1988.tb11509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne C., Mironneau C., Mironneau J., Savineau J. P. Contractions of rat uterine smooth muscle induced by acetylcholine and angiotensin II in Ca2+-free medium. Br J Pharmacol. 1984 Feb;81(2):317–326. doi: 10.1111/j.1476-5381.1984.tb10081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel E., Deitmer P., Noack T. Suppression of steady membrane currents by acetylcholine in single smooth muscle cells of the guinea-pig gastric fundus. J Physiol. 1991 Jan;432:259–282. doi: 10.1113/jphysiol.1991.sp018384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Peralta E., Clapham D. Diverse functions of muscarinic acetylcholine receptor subtypes. Trends Pharmacol Sci. 1989 Dec;Suppl:34–38. [PubMed] [Google Scholar]
- Lee T. P., Kuo J. F., Greengard P. Role of muscarinic cholinergic receptors in regulation of guanosine 3':5'-cyclic monophosphate content in mammalian brain, heart muscle, and intestinal smooth muscle. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3287–3291. doi: 10.1073/pnas.69.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1988 Feb;62(2):375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Straughan D. W. The release of acetylcholine from mammalian motor nerve endings. Br J Pharmacol Chemother. 1960 Sep;15(3):417–424. doi: 10.1111/j.1476-5381.1960.tb01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy V. C., Triggle C. R., Triggle D. J. The effects of lanthanum and thulium on the mechanical responses of rat vas deferens. J Physiol. 1976 Jan;254(1):55–62. doi: 10.1113/jphysiol.1976.sp011220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed M. M., Parekh A. B., Tomita T. Receptors involved in mechanical responses to catecholamines in the circular muscle of guinea-pig stomach treated with meclofenamate. Br J Pharmacol. 1990 Dec;101(4):809–814. doi: 10.1111/j.1476-5381.1990.tb14162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]


