Abstract
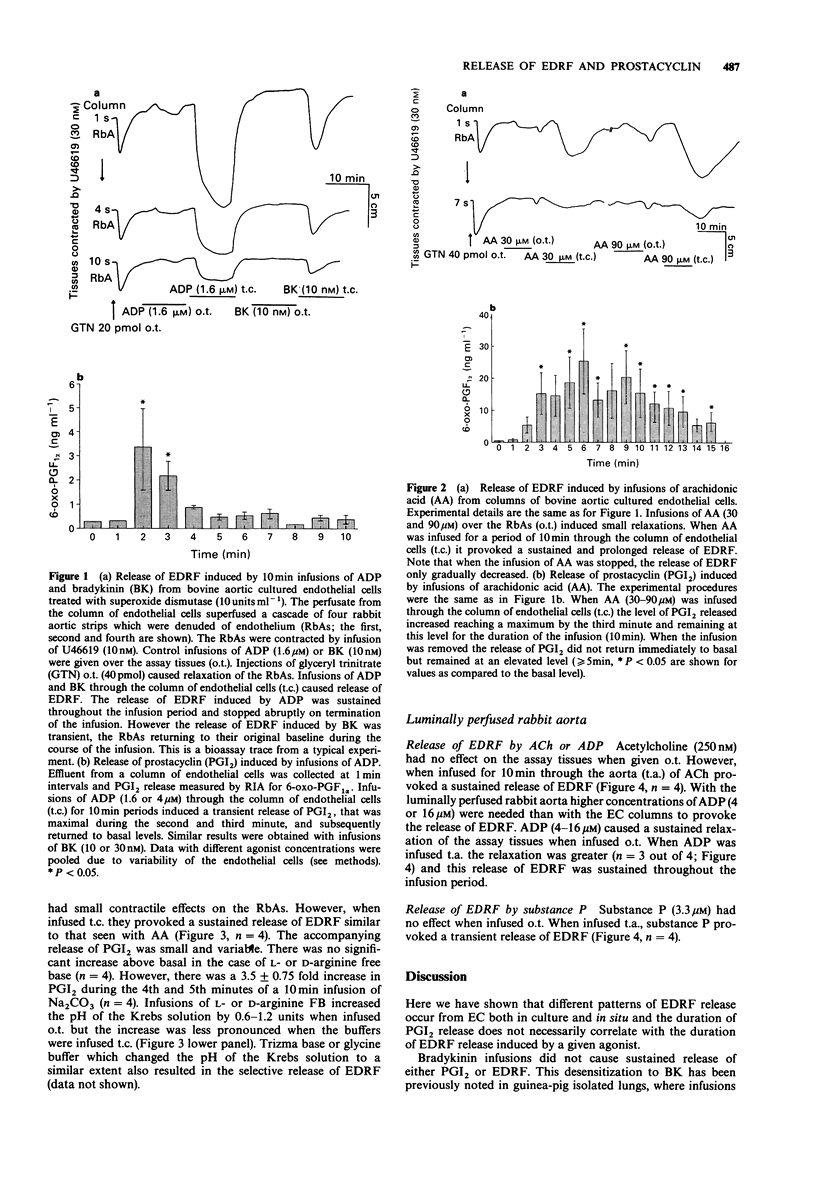
1. Release of endothelium derived relaxing factor (EDRF) and prostacyclin (PGI2) from endothelial cells (EC) cultured from bovine aortae was measured by bioassay and radioimmunoassay, respectively, during infusions (10 min) of bradykinin (BK), adenosine diphosphate (ADP), arachidonic acid (AA), alkaline buffers and the free-bases (FB) of L-arginine or D-arginine. Release of EDRF from the luminally perfused rabbit aorta was also measured during infusions (10 min) of acetylcholine (ACh), substance P and ADP. 2. Bradykinin (10 or 30 nM) infused through the column of EC induced release of both EDRF and PGI2, neither of which was maintained for the duration of the infusion. 3. ADP (1.6 or 4 microM) infused through the column of EC induced release of a EDRF which was maintained for the duration of the infusion and a release of PGI2 which lasted for a much shorter period. 4. Arachidonic acid (30 or 90 microM) infused through the column of EC caused a sustained release of EDRF and PGI2, both of which outlasted the infusion of AA. 5. L-Arginine FB, D-arginine FB or alkaline buffer infused through the column of EC released EDRF, but only small amounts of PGI2. The release of EDRF outlasted the period of infusion and was due to an increase in the pH of the Krebs solution perfusing the EC. 6. Infusions of ACh (0.25-1 microM) or ADP (4-16 microM) caused a sustained release of EDRF from the luminally-perfused rabbit aorta, whereas infusion of substance P (3.3-10 microM) caused only a transient release of EDRF. 7. These results show that distinct patterns of EDRF release exist to different agonists in both cultured and in situ EC, and that EDRF and PGI2 do not necessarily follow the same time course of release. Furthermore, sustained release of EDRF does not require the constant infusion of the precursor, L-arginine, whereas sustained release of PGI2 only occurs when AA, the precursor of PGI2, is present in the extracellular medium.
Full text
PDF

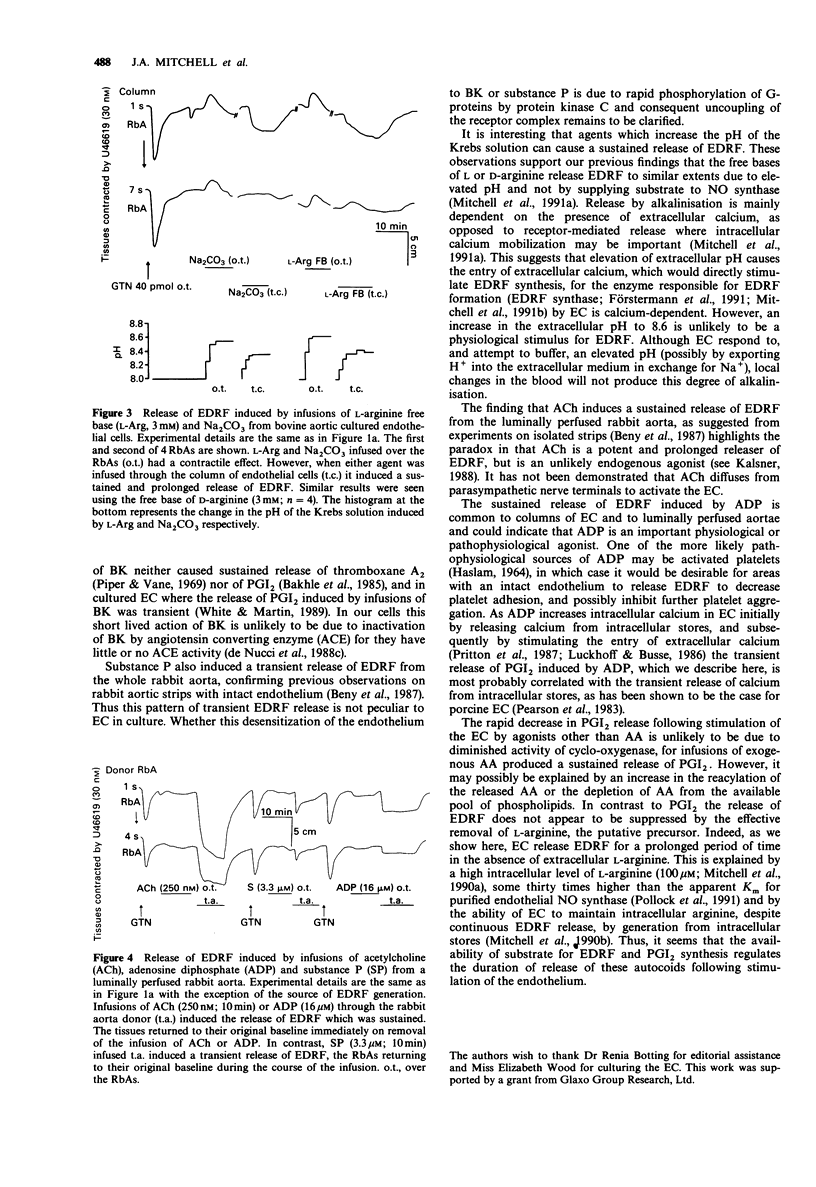

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhle Y. S., Moncada S., de Nucci G., Salmon J. A. Differential release of eicosanoids by bradykinin, arachidonic acid and calcium ionophore A23187 in guinea-pig isolated perfused lung. Br J Pharmacol. 1985 Sep;86(1):55–62. doi: 10.1111/j.1476-5381.1985.tb09434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C. Electrophysiological and mechanical effects of substance P and acetylcholine on rabbit aorta. J Physiol. 1988 Apr;398:277–289. doi: 10.1113/jphysiol.1988.sp017042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Cherry P. D., Zawadzki J. V., Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Pollock J. S., Schmidt H. H., Heller M., Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Botting R. M., Vane J. R. Mediators produced by the endothelial cell. Hypertension. 1988 Dec;12(6):530–548. doi: 10.1161/01.hyp.12.6.530. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLAM R. J. ROLE OF ADENOSINE DIPHOSPHATE IN THE AGGREGATION OF HUMAN BLOOD-PLATELETS BY THROMBIN AND BY FATTY ACIDS. Nature. 1964 May 23;202:765–768. doi: 10.1038/202765a0. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Flower R. J., Vane J. R. A new approach to anti-inflammatory drugs. Biochem Pharmacol. 1979 Jun 15;28(12):1959–1961. doi: 10.1016/0006-2952(79)90651-8. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S. Cholinergic constriction in the general circulation and its role in coronary artery spasm. Circ Res. 1989 Aug;65(2):237–257. doi: 10.1161/01.res.65.2.237. [DOI] [PubMed] [Google Scholar]
- Kondo K., Mitchell J. A., de Nucci G., Vane J. R. Simultaneous measurement of endothelium-derived relaxing factor by bioassay and guanylate cyclase stimulation. Br J Pharmacol. 1989 Oct;98(2):630–636. doi: 10.1111/j.1476-5381.1989.tb12637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. A., Förstermann U., Warner T. D., Pollock J. S., Schmidt H. H., Heller M., Murad F. Endothelial cells have a particulate enzyme system responsible for EDRF formation: measurement by vascular relaxation. Biochem Biophys Res Commun. 1991 May 15;176(3):1417–1423. doi: 10.1016/0006-291x(91)90444-c. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Hecker M., Anggård E. E., Vane J. R. Cultured endothelial cells maintain their L-arginine level despite the continuous release of EDRF. Eur J Pharmacol. 1990 Jul 17;182(3):573–576. doi: 10.1016/0014-2999(90)90058-e. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Hecker M., Vane J. R. The generation of L-arginine in endothelial cells is linked to the release of endothelium-derived relaxing factor. Eur J Pharmacol. 1990 Feb 6;176(2):253–254. doi: 10.1016/0014-2999(90)90541-d. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., de Nucci G., Warner T. D., Vane J. R. Alkaline buffers release EDRF from bovine cultured aortic endothelial cells. Br J Pharmacol. 1991 Jun;103(2):1295–1302. doi: 10.1111/j.1476-5381.1991.tb09783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- PATON W. D. A pendulum auxotonic lever. J Physiol. 1957 Jul 11;137(2):35P–356. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Slakey L. L., Gordon J. L. Stimulation of prostaglandin production through purinoceptors on cultured porcine endothelial cells. Biochem J. 1983 Jul 15;214(1):273–276. doi: 10.1042/bj2140273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P. J., Vane J. R. Release of additional factors in anaphylaxis and its antagonism by anti-inflammatory drugs. Nature. 1969 Jul 5;223(5201):29–35. doi: 10.1038/223029a0. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYHAGE R., SAMUELSSON B. THE ORIGIN OF OXYGEN INCORPORATED DURING THE BIOSYNTHESIS OF PROSTAGLANDIN E-1. Biochem Biophys Res Commun. 1965 Apr 23;19:279–282. doi: 10.1016/0006-291x(65)90454-7. [DOI] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F., Griffith O. W., Feldman P. L., Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991 Apr 5;266(10):6259–6263. [PubMed] [Google Scholar]
- VANE J. R. THE USE OF ISOLATED ORGANS FOR DETECTING ACTIVE SUBSTANCES IN THE CIRCULATING BLOOD. Br J Pharmacol Chemother. 1964 Oct;23:360–373. doi: 10.1111/j.1476-5381.1964.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner T. D., Mitchell J. A., de Nucci G., Vane J. R. Endothelin-1 and endothelin-3 release EDRF from isolated perfused arterial vessels of the rat and rabbit. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S85–S102. doi: 10.1097/00005344-198900135-00021. [DOI] [PubMed] [Google Scholar]
- White D. G., Martin W. Differential control and calcium-dependence of production of endothelium-derived relaxing factor and prostacyclin by pig aortic endothelial cells. Br J Pharmacol. 1989 Jul;97(3):683–690. doi: 10.1111/j.1476-5381.1989.tb12004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nucci G., Warner T., Vane J. R. Effect of captopril on the bradykinin-induced release of prostacyclin from guinea-pig lungs and bovine aortic endothelial cells. Br J Pharmacol. 1988 Nov;95(3):783–788. doi: 10.1111/j.1476-5381.1988.tb11705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


