Abstract
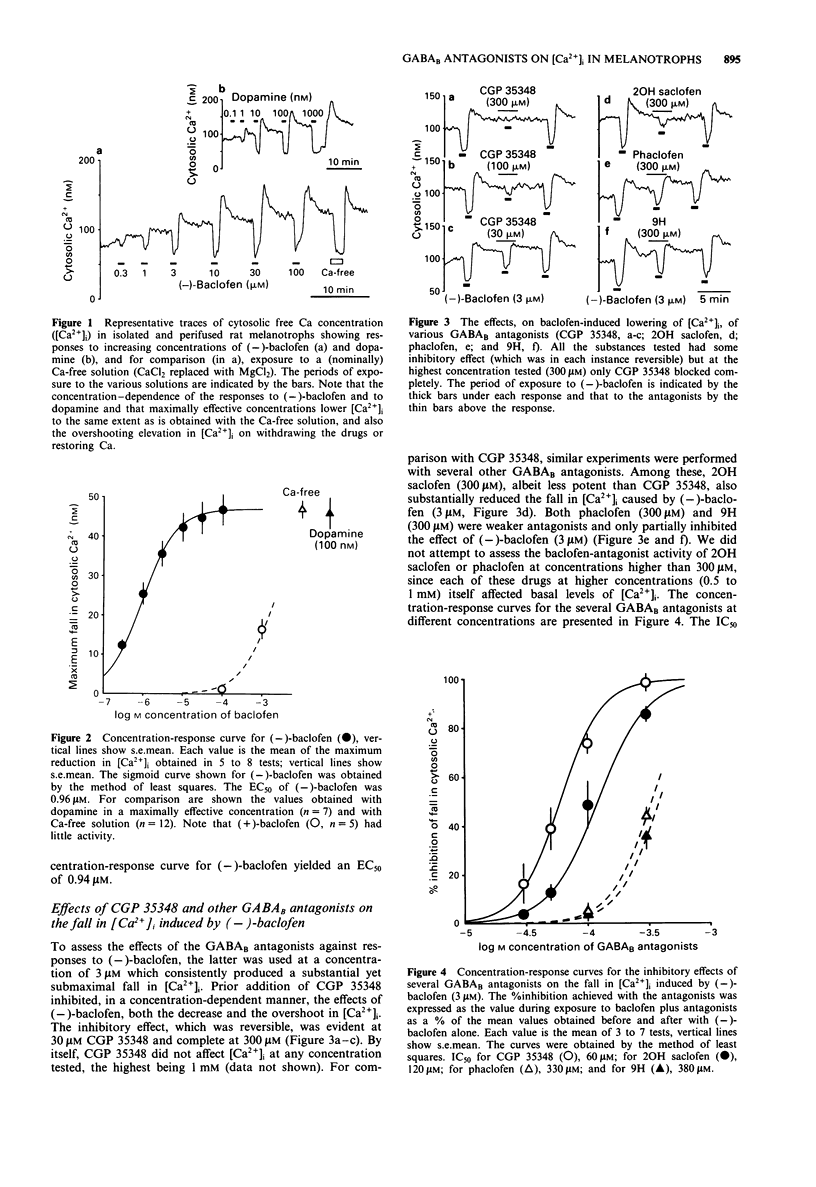
1. The purpose of the present experiments was to assess the activities of GABAB receptor antagonists in mammalian isolated melanotrophs. 2. Cytosolic free Ca concentration ([Ca2+]i) in rat melanotrophs in primary culture was monitored with the fluorescent probe, fura-2. 3. (-)-Baclofen lowered [Ca2+]i in a concentration-dependent manner with an EC50 of 0.96 microM. The reduction in [Ca2+]i produced by (-)-baclofen at a maximally effective concentration (100 microM) was similar to that produced by the classic transmitter inhibitory to melanotroph secretion, dopamine, at a corresponding concentration (100 nM), or by perifusion with a nominally Ca-free solution. 4. The GABAB receptor antagonists, 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP 35348), 2-hydroxy saclofen, phaclofen and 4-amino-3-(5-methoxybenzo[b]furan-2-yl) butanoic acid (9H), had inhibitory effects on the reduction in [Ca2+]i produced by (-)-baclofen (3 microM). Of the antagonists tested, CGP 35348 was the most potent with an IC50 of 60 microM, compared to 120 to 400 microM for the others. CGP 35348 acted competitively. 5. CGP 35348 alone had no effect on basal [Ca2+]i, or on the changes in [Ca2+]i produced by dopamine (10 nM) or the specific GABAA receptor agonist, muscimol (10 microM). 6. The evidence indicates that of the antagonists tested, CGP 35348 offers the greatest promise for pharmacological analysis of the functional significance of the GABAB receptors in melanotrophs.
Full text
PDF

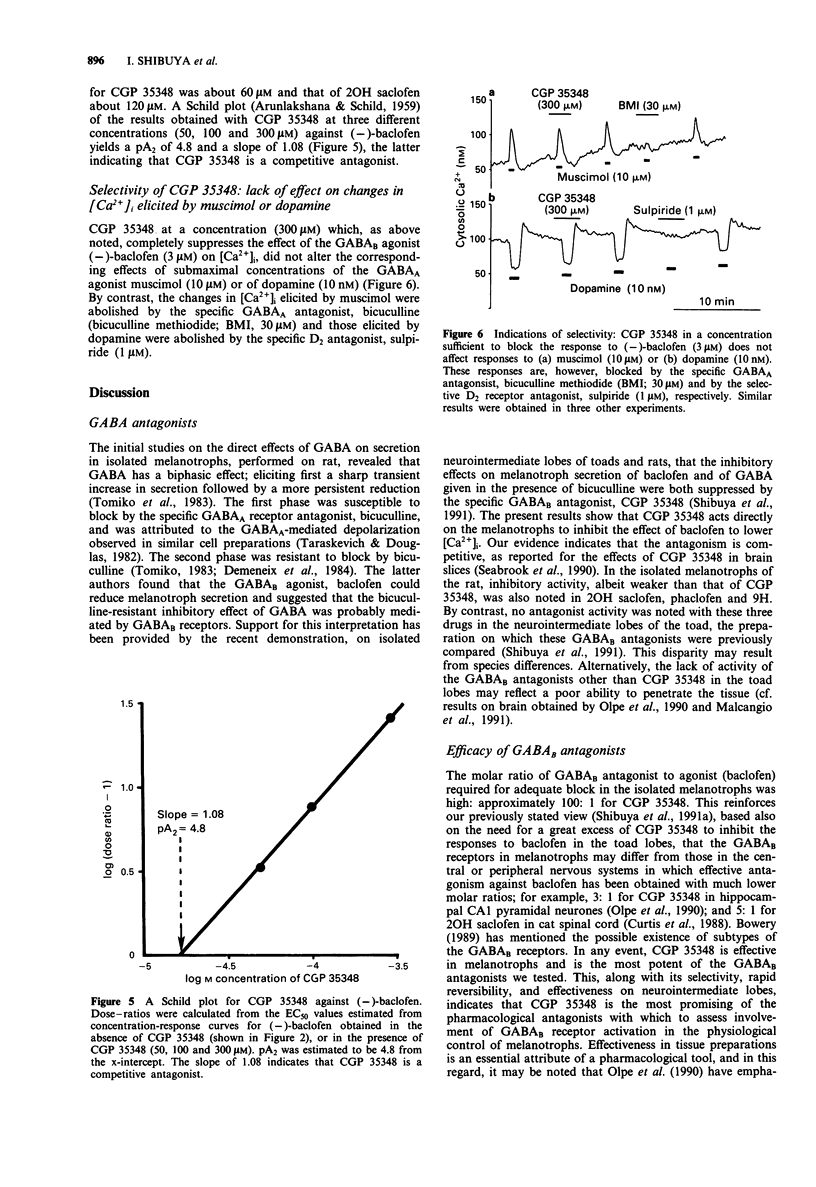


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. T., Curtis D. R., Debaert M., Vaccher C., Berthelot P. Baclofen antagonism by 4-amino-3-(5-methoxybenzo[b]furan-2-yl) butanoic acid in the cat spinal cord. Neurosci Lett. 1989 May 22;100(1-3):292–294. doi: 10.1016/0304-3940(89)90701-5. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989 Oct;10(10):401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Gynther B. D., Beattie D. T., Kerr D. I., Prager R. H. Baclofen antagonism by 2-hydroxy-saclofen in the cat spinal cord. Neurosci Lett. 1988 Sep 23;92(1):97–101. doi: 10.1016/0304-3940(88)90749-5. [DOI] [PubMed] [Google Scholar]
- Demeneix B. A., Desaulles E., Feltz P., Loeffler J. P. Dual population of GABAA and GABAB receptors in rat pars intermedia demonstrated by release of alpha MSH caused by barium ions. Br J Pharmacol. 1984 May;82(1):183–190. doi: 10.1111/j.1476-5381.1984.tb16457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Holzbauer M., Racké K. The dopaminergic innervation of the intermediate lobe and of the neural lobe of the pituitary gland. Med Biol. 1985;63(3):97–116. [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Johnston G. A., Abbenante J., Prager R. H. 2-Hydroxy-saclofen: an improved antagonist at central and peripheral GABAB receptors. Neurosci Lett. 1988 Sep 23;92(1):92–96. doi: 10.1016/0304-3940(88)90748-3. [DOI] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Kongsamut S., Shibuya I., Douglas W. W. Why are several inhibitory transmitters present in the innervation of pituitary melanotrophs? Actions and interactions of dopamine, GABA and neuropeptide Y on secretion from neurointermediate lobes of Xenopus laevis. Neuroendocrinology. 1991 Dec;54(6):599–606. doi: 10.1159/000125966. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Pittman Q. J. Novel synaptic responses mediated by dopamine and gamma-aminobutyric acid in neuroendocrine cells of the intermediate pituitary. Neurosci Lett. 1986 Feb 14;64(1):35–40. doi: 10.1016/0304-3940(86)90659-2. [DOI] [PubMed] [Google Scholar]
- Malcangio M., Ghelardini C., Giotti A., Malmberg-Aiello P., Bartolini A. CGP 35348, a new GABAB antagonist, prevents antinociception and muscle-relaxant effect induced by baclofen. Br J Pharmacol. 1991 Jun;103(2):1303–1308. doi: 10.1111/j.1476-5381.1991.tb09784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E. F., Taraskevich P. S., Douglas W. W. Cytosolic Ca2+ in melanotrophs: pharmacological insights into regulatory influences of electrical activity and ion channels. Endocrinology. 1990 Feb;126(2):754–758. doi: 10.1210/endo-126-2-754. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Mugnaini E., Tappaz M. L., Weise V. K., Dahl A. L., Schmechel D. E., Kopin I. J. Central GABAergic innervation of neurointermediate pituitary lobe: biochemical and immunocytochemical study in the rat. Proc Natl Acad Sci U S A. 1982 Jan;79(2):675–679. doi: 10.1073/pnas.79.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe H. R., Karlsson G., Pozza M. F., Brugger F., Steinmann M., Van Riezen H., Fagg G., Hall R. G., Froestl W., Bittiger H. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990 Oct 2;187(1):27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- Sakaue M., Saito N., Taniguchi H., Baba S., Tanaka C. Immunohistochemical localization of gamma-aminobutyric acid in the rat pituitary gland and related hypothalamic regions. Brain Res. 1988 Apr 19;446(2):343–353. doi: 10.1016/0006-8993(88)90893-1. [DOI] [PubMed] [Google Scholar]
- Schimchowitsch S., Vuillez P., Tappaz M. L., Klein M. J., Stoeckel M. E. Systematic presence of GABA-immunoreactivity in the tubero-infundibular and tubero-hypophyseal dopaminergic axonal systems: an ultrastructural immunogold study on several mammals. Exp Brain Res. 1991;83(3):575–586. doi: 10.1007/BF00229836. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Howson W., Lacey M. G. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990 Dec;101(4):949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya I., Kongsamut S., Douglas W. W. Studies on pituitary melanotrophs reveal the novel GABAB antagonist CGP 35-348 to be the first such compound effective on endocrine cells. Proc Biol Sci. 1991 Feb 22;243(1307):129–137. doi: 10.1098/rspb.1991.0021. [DOI] [PubMed] [Google Scholar]
- Stoeckel M. E., Tappaz M., Hindelang C., Seweryn C., Porte A. Opposite effects of monosodium glutamate on the dopaminergic and GABAergic innervations of the median eminence and the intermediate lobe in the mouse. Neurosci Lett. 1985 May 23;56(3):249–255. doi: 10.1016/0304-3940(85)90251-4. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Dopamine (D2) or gamma-aminobutyric acid (GABAB) receptor activation hyperpolarizes rat melanotrophs and pertussis toxin blocks these responses and the accompanying fall in [Ca2+]i. Neurosci Lett. 1990 May 4;112(2-3):205–209. doi: 10.1016/0304-3940(90)90204-m. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. GABA directly affects electrophysiological properties of pituitary pars intermedia cells. Nature. 1982 Oct 21;299(5885):733–734. doi: 10.1038/299733a0. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Pharmacological and ionic features of gamma-aminobutyric acid receptors influencing electrical properties of melanotrophs isolated from the rat pars intermedia. Neuroscience. 1985 Jan;14(1):301–308. doi: 10.1016/0306-4522(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. Effects of veratridine, tetrodotoxin and other drugs that alter electrical behaviour on secretion of melanocyte-stimulating hormone from melanotrophs of the pituitary pars intermedia. Neuroscience. 1984 Aug;12(4):1223–1228. doi: 10.1016/0306-4522(84)90016-2. [DOI] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. GABA acts directly on cells of pituitary pars intermedia to alter hormone output. Nature. 1983 Feb 24;301(5902):706–707. doi: 10.1038/301706a0. [DOI] [PubMed] [Google Scholar]
- Verburg-van Kemenade B. M., Jenks B. G., Lenssen F. J., Vaudry H. Characterization of gamma-aminobutyric acid receptors in the neurointermediate lobe of the amphibian Xenopus laevis. Endocrinology. 1987 Feb;120(2):622–628. doi: 10.1210/endo-120-2-622. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y. GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology. 1982 Feb;34(2):117–125. doi: 10.1159/000123288. [DOI] [PubMed] [Google Scholar]
- Vuillez P., Pérez S. C., Stoeckel M. E. Colocalization of GABA and tyrosine hydroxylase immunoreactivities in the axons innervating the neurointermediate lobe of the rat pituitary: an ultrastructural immunogold study. Neurosci Lett. 1987 Aug 18;79(1-2):53–58. doi: 10.1016/0304-3940(87)90671-9. [DOI] [PubMed] [Google Scholar]
- Williams P. J., MacVicar B. A., Pittman Q. J. Identification of a GABA-activated chloride-mediated synaptic potential in rat pars intermedia. Brain Res. 1989 Mar 27;483(1):130–134. doi: 10.1016/0006-8993(89)90043-7. [DOI] [PubMed] [Google Scholar]


