Abstract
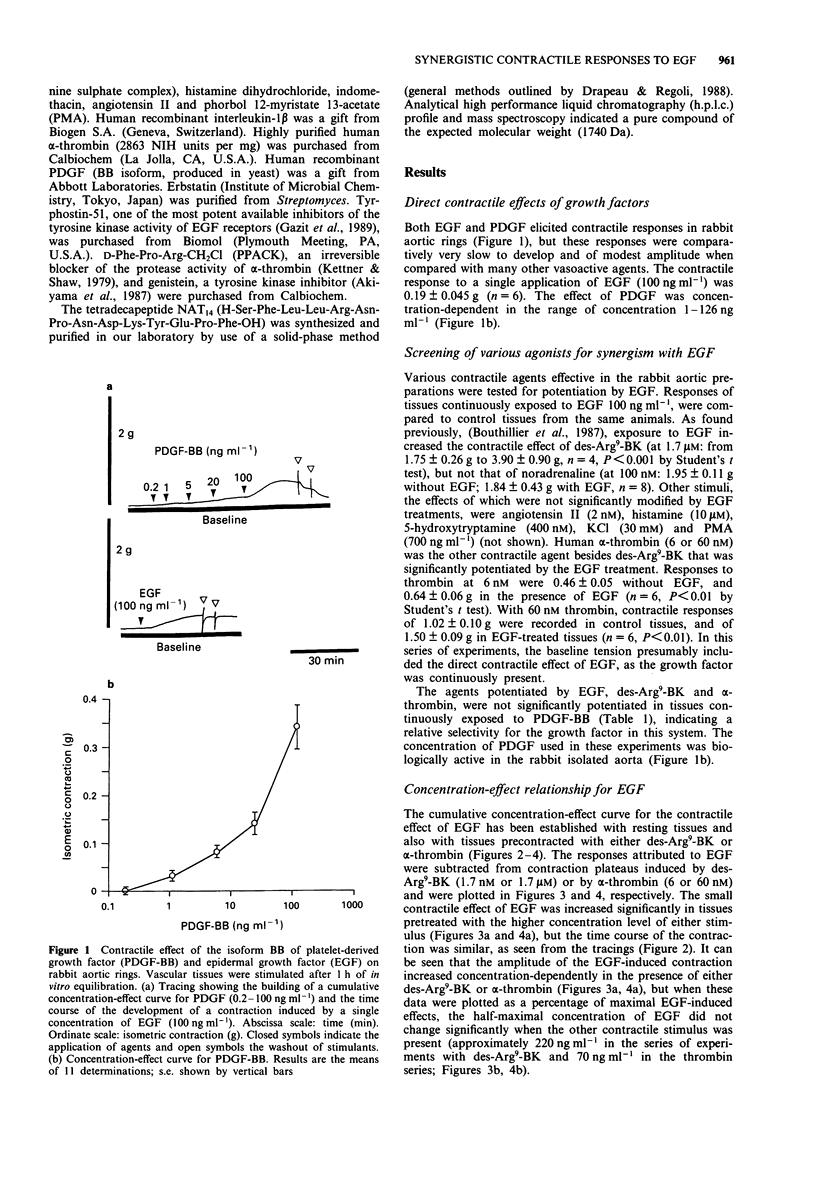
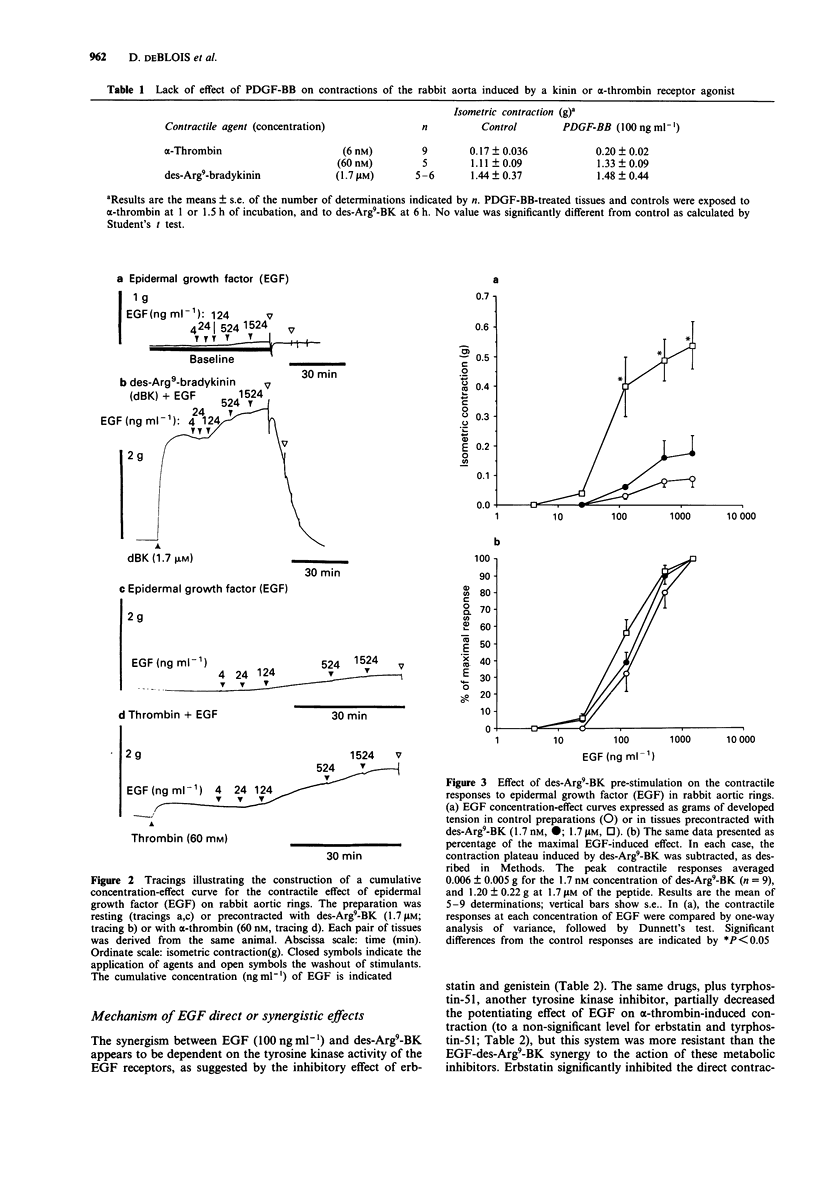
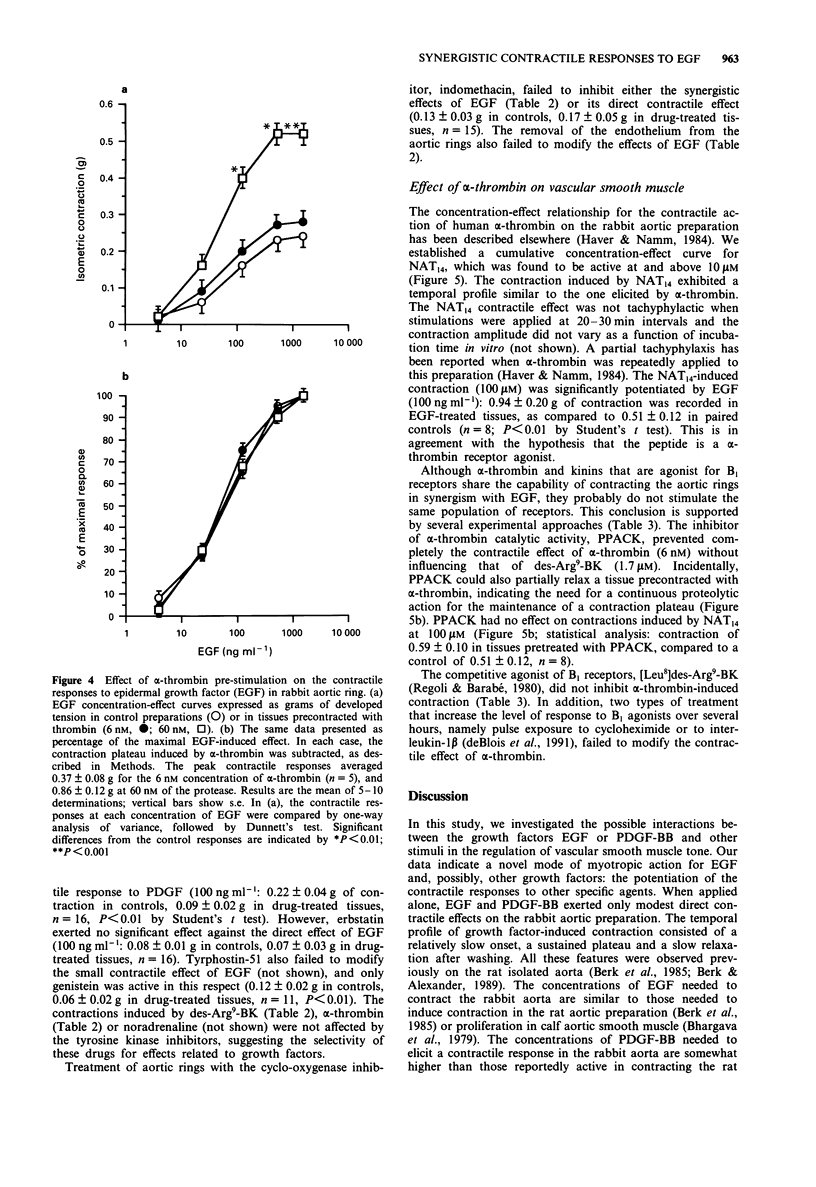
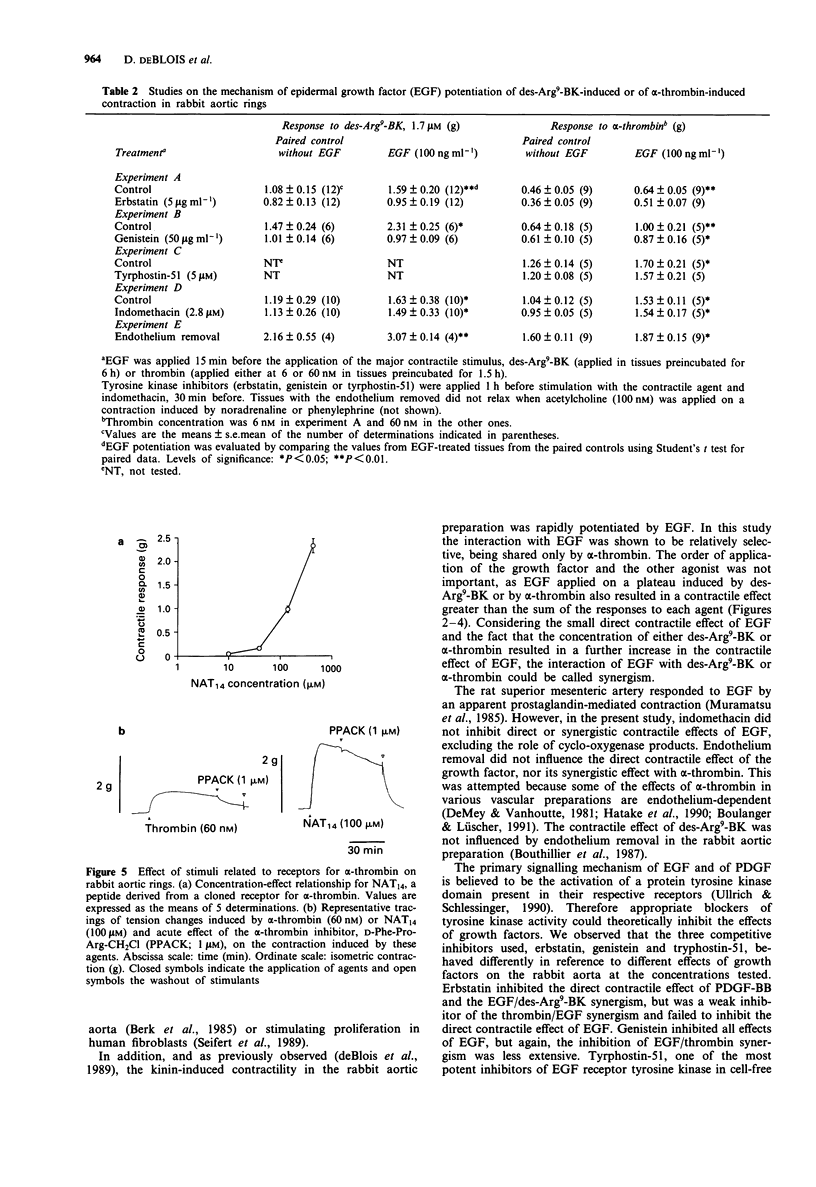
1. Rabbit aortic rings were used to test the possible contractile effects of growth factors and their interaction with other stimuli. A rapid potentiation of kinin-induced contraction by epidermal growth factor (EGF) has been previously observed in this preparation. 2. EGF (5-1500 ng ml-1) and the isoform BB of platelet-derived growth factor (PDGF-BB; 1-126 ng ml-1) exerted modest but sustained contractile effects in rabbit aortic rings. 3. EGF pretreatment (100 ng ml-1) potentiated the contractile responses to des-Arg9-bradykinin (des-Arg9-BK), an agonist of the B1 receptors for kinin found in this preparation, and to human alpha-thrombin but not to several other contractile stimuli. The interaction appeared also relatively selective for the growth factor, because PDGF-BB pretreatment potentiated neither des-Arg9-BK nor alpha-thrombin-induced contraction. 4. EGF, applied on a contraction plateau induced by des-Arg9-BK or alpha-thrombin, exerted a synergistic contractile effect, with a time course and a half-maximal concentration for EGF-induced contraction similar to the ones recorded in resting tissues (between 67 and 220 ng ml-1, depending on the series of experiments). 5. The direct or synergistic contractile effects of EGF were not modified by the removal of the endothelium or by treatment with indomethacin. However, the tyrosine kinase inhibitors, erbstatin or genistein, inhibited the synergistic effect of EGF with des-Arg9-BK. The small direct contractile effect of EGF was significantly reduced by genistein. The synergistic effect of EGF with alpha-thrombin was comparatively more resistant to the tested tyrosine kinase inhibitors.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Aronson D. L., Stevan L., Ball A. P., Franza B. R., Jr, Finlayson J. S. Generation of the combined prothrombin activation peptide (F1-2) during the clotting of blood and plasma. J Clin Invest. 1977 Dec;60(6):1410–1418. doi: 10.1172/JCI108902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beny J. L., Brunet P., Huggel H. Interaction of bradykinin and des-Arg9-bradykinin with isolated pig coronary arteries: mechanical and electrophysiological events. Regul Pept. 1987 Apr;17(4):181–190. doi: 10.1016/0167-0115(87)90061-9. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Alexander R. W. Vasoactive effects of growth factors. Biochem Pharmacol. 1989 Jan 15;38(2):219–225. doi: 10.1016/0006-2952(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Brock T. A., Webb R. C., Taubman M. B., Atkinson W. J., Gimbrone M. A., Jr, Alexander R. W. Epidermal growth factor, a vascular smooth muscle mitogen, induces rat aortic contraction. J Clin Invest. 1985 Mar;75(3):1083–1086. doi: 10.1172/JCI111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk B. C., Taubman M. B., Cragoe E. J., Jr, Fenton J. W., 2nd, Griendling K. K. Thrombin signal transduction mechanisms in rat vascular smooth muscle cells. Calcium and protein kinase C-dependent and -independent pathways. J Biol Chem. 1990 Oct 5;265(28):17334–17340. [PubMed] [Google Scholar]
- Bhargava G., Rifas L., Makman M. H. Presence of epidermal growth factor receptors and influence of epidermal growth factor on proliferation and aging in cultured smooth muscle cells. J Cell Physiol. 1979 Aug;100(2):365–374. doi: 10.1002/jcp.1041000217. [DOI] [PubMed] [Google Scholar]
- Boulanger C. M., Lüscher T. F. Hirudin and nitrates inhibit the thrombin-induced release of endothelin from the intact porcine aorta. Circ Res. 1991 Jun;68(6):1768–1772. doi: 10.1161/01.res.68.6.1768. [DOI] [PubMed] [Google Scholar]
- Bouthillier J., Deblois D., Marceau F. Studies on the induction of pharmacological responses to des-Arg9-bradykinin in vitro and in vivo. Br J Pharmacol. 1987 Oct;92(2):257–264. doi: 10.1111/j.1476-5381.1987.tb11319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. D., Deth R. C., Payne R. A., Honeyman T. W. Phosphoinositide hydrolysis is correlated with agonist-induced calcium flux and contraction in the rabbit aorta. Eur J Pharmacol. 1985 Oct 8;116(1-2):129–136. doi: 10.1016/0014-2999(85)90193-1. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Role of the intima in the relaxation of the canine femoral artery caused by thrombin. Arch Int Pharmacodyn Ther. 1981 Apr;250(2):314–315. [PubMed] [Google Scholar]
- Deblois D., Bouthillier J., Marceau F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit isolated aorta to des-Arg9-bradykinin. Br J Pharmacol. 1988 Apr;93(4):969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin. Ann N Y Acad Sci. 1986;485:5–15. doi: 10.1111/j.1749-6632.1986.tb34563.x. [DOI] [PubMed] [Google Scholar]
- Fischell T. A., Derby G., Tse T. M., Stadius M. L. Coronary artery vasoconstriction routinely occurs after percutaneous transluminal coronary angioplasty. A quantitative arteriographic analysis. Circulation. 1988 Dec;78(6):1323–1334. doi: 10.1161/01.cir.78.6.1323. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Kim J. W., Machesky L. M., Rhee S. G., Pollard T. D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991 Mar 8;251(4998):1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Brown K. D., Birdwell C. R., Zetter B. R. Control of proliferation of human vascular endothelial cells. Characterization of the response of human umbilical vein endothelial cells to fibroblast growth factor, epidermal growth factor, and thrombin. J Cell Biol. 1978 Jun;77(3):774–788. doi: 10.1083/jcb.77.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatake K., Kakishita E., Wakabayashi I., Sakiyama N., Hishida S. Effect of aging on endothelium-dependent vascular relaxation of isolated human basilar artery to thrombin and bradykinin. Stroke. 1990 Jul;21(7):1039–1043. doi: 10.1161/01.str.21.7.1039. [DOI] [PubMed] [Google Scholar]
- Hatton M. W., Moar S. L., Richardson M. Deendothelialization in vivo initiates a thrombogenic reaction at the rabbit aorta surface. Correlation of uptake of fibrinogen and antithrombin III with thrombin generation by the exposed subendothelium. Am J Pathol. 1989 Sep;135(3):499–508. [PMC free article] [PubMed] [Google Scholar]
- Haver V. M., Namm D. H. Characterization of the thrombin-induced contraction of vascular smooth muscle. Blood Vessels. 1984;21(2):53–63. [PubMed] [Google Scholar]
- Imoto M., Umezawa K., Isshiki K., Kunimoto S., Sawa T., Takeuchi T., Umezawa H. Kinetic studies of tyrosine kinase inhibition by erbstatin. J Antibiot (Tokyo) 1987 Oct;40(10):1471–1473. doi: 10.7164/antibiotics.40.1471. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Kalsner S., Richards R. Coronary arteries of cardiac patients are hyperreactive and contain stores of amines: a mechanism for coronary spasm. Science. 1984 Mar 30;223(4643):1435–1437. doi: 10.1126/science.6701530. [DOI] [PubMed] [Google Scholar]
- Kettner C., Shaw E. D-Phe-Pro-ArgCH2C1-A selective affinity label for thrombin. Thromb Res. 1979;14(6):969–973. doi: 10.1016/0049-3848(79)90014-8. [DOI] [PubMed] [Google Scholar]
- Ku D. D. Unmasking of thrombin vasoconstriction in isolated perfused dog hearts after intracoronary infusion of air embolus. J Pharmacol Exp Ther. 1987 Nov;243(2):571–576. [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- Marceau F., Petitclerc E., DeBlois D., Pradelles P., Poubelle P. E. Human interleukin-1 induces a rapid relaxation of the rabbit isolated mesenteric artery. Br J Pharmacol. 1991 Jun;103(2):1367–1372. doi: 10.1111/j.1476-5381.1991.tb09795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. W., Kruskal B. A., Maxfield F. R. Simultaneous addition of EGF prolongs the increase in cytosolic free calcium seen in response to bradykinin in NRK-49F cells. J Cell Physiol. 1988 Sep;136(3):519–525. doi: 10.1002/jcp.1041360318. [DOI] [PubMed] [Google Scholar]
- Muramatsu I., Hollenberg M. D., Lederis K. Vascular actions of epidermal growth factor-urogastrone: possible relationship to prostaglandin production. Can J Physiol Pharmacol. 1985 Aug;63(8):994–999. doi: 10.1139/y85-164. [DOI] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R., Santone K., Melder D., Oakes S. G., Abraham R., Powis G. An increase in intracellular free Ca2+ associated with serum-free growth stimulation of Swiss 3T3 fibroblasts by epidermal growth factor in the presence of bradykinin. J Biol Chem. 1988 Dec 5;263(34):18030–18035. [PubMed] [Google Scholar]
- Pandiella A., Beguinot L., Vicentini L. M., Meldolesi J. Transmembrane signalling at the epidermal growth factor receptor. Trends Pharmacol Sci. 1989 Oct;10(10):411–414. doi: 10.1016/0165-6147(89)90190-9. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Meldolesi J. Reinforcement of signal generation at B2 bradykinin receptors by insulin, epidermal growth factors, and other growth factors. J Biol Chem. 1989 Feb 25;264(6):3122–3130. [PubMed] [Google Scholar]
- Powis G. Signalling targets for anticancer drug development. Trends Pharmacol Sci. 1991 May;12(5):188–194. doi: 10.1016/0165-6147(91)90545-4. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Shuman M. A. Thrombin-cellular interactions. Ann N Y Acad Sci. 1986;485:228–239. doi: 10.1111/j.1749-6632.1986.tb34585.x. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- deBlois D., Bouthillier J., Marceau F. Pharmacological modulation of the up-regulated responses to des-Arg9-bradykinin in vivo and in vitro. Immunopharmacology. 1989 May-Jun;17(3):187–198. doi: 10.1016/0162-3109(89)90047-7. [DOI] [PubMed] [Google Scholar]
- deBlois D., Bouthillier J., Marceau F. Pulse exposure to protein synthesis inhibitors enhances vascular responses to des-Arg9-bradykinin: possible role of interleukin-1. Br J Pharmacol. 1991 May;103(1):1057–1066. doi: 10.1111/j.1476-5381.1991.tb12300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


