Abstract
1. The effects of ryanodine and caffeine on intracellular free Ca2+ concentration ([Ca2+]i) were studied by use of fura-2 microfluorometry in single smooth muscle cells freshly dispersed from bovine and porcine coronary artery. 2. Bovine and porcine cells demonstrated similar sensitivities to 10 min of exposure to ryanodine in physiological salt solution (PSS), as determined by comparable dose-dependent decreases in the subsequent [Ca2+]i transient induced by 5 mM caffeine. 3. Ryanodine (10 microM) caused a significant increase in [Ca2+]i to a plateau level 27 +/- 3% and 38 +/- 4% above baseline [Ca2+]i (baseline [Ca2+]i = [Ca2+]i at 0 min) in porcine and bovine cells, respectively, when bathed in PSS. In bovine cells the time required to reach 1/2 the plateau level was only 3 min versus 6 min for porcine cells. 4. The ryanodine-induced plateau increase in [Ca2+]i was 35 +/- 5% above baseline for bovine cells bathed in 0 Ca PSS (PSS including 10 microM EGTA with no added Ca2+), but only 7 +/- 3% above baseline in porcine cells during 10 min exposure to 10 microM ryanodine. In bovine cells [Ca2+]i showed proportional increases when extracellular Ca2+ was increased from the normal 2 mM Ca2+ PSS to 5 and 10 mM. 5. Cells pretreated with caffeine in 0 Ca PSS, which depleted the caffeine-sensitive sarcoplasmic reticulum Ca2+ store, showed no increase in [Ca2+]i when challenged with 10 microM ryanodine. The ryanodine-associated increase in [Ca2+]i, which was sustained in 0 Ca PSS during the 10 min ryanodine exposure in cells not pretreated with caffeine, suggests that ryanodine releases Ca2+ from the sarcoplasmic reticulum, but also inhibits Ca2+ efflux.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


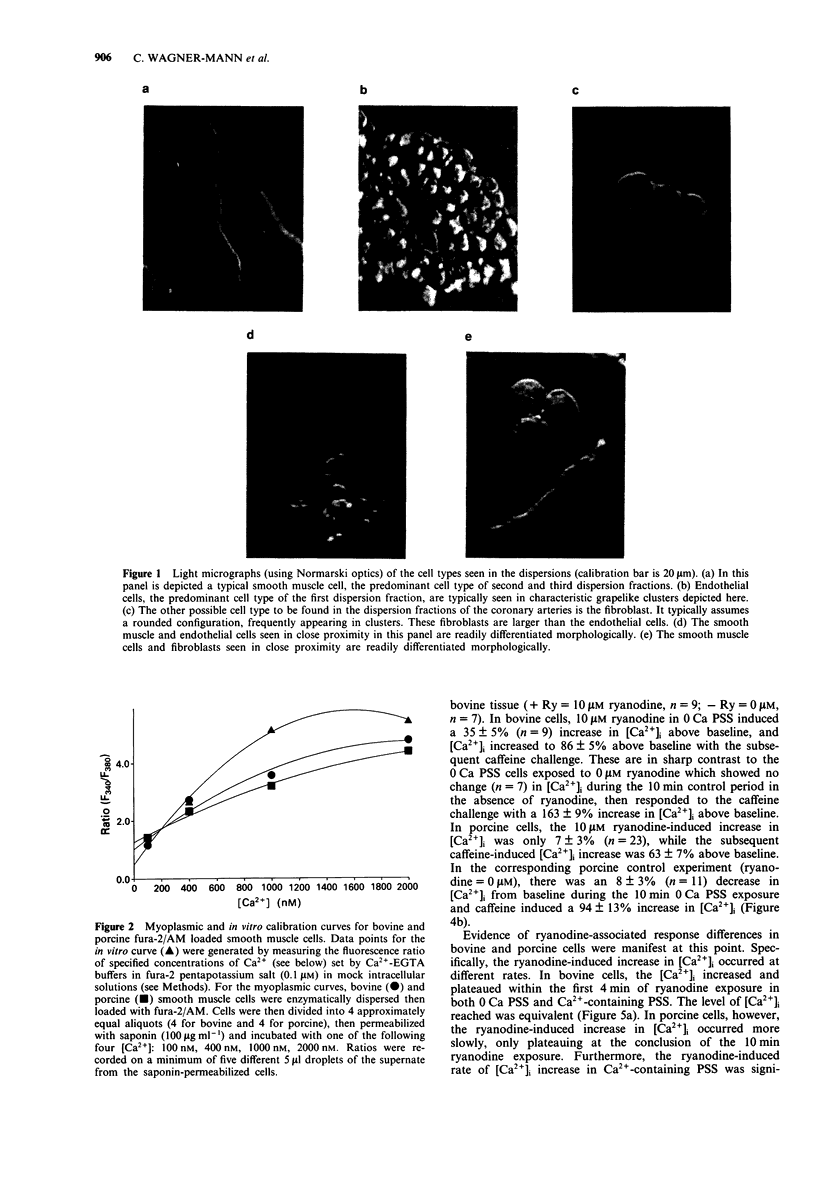
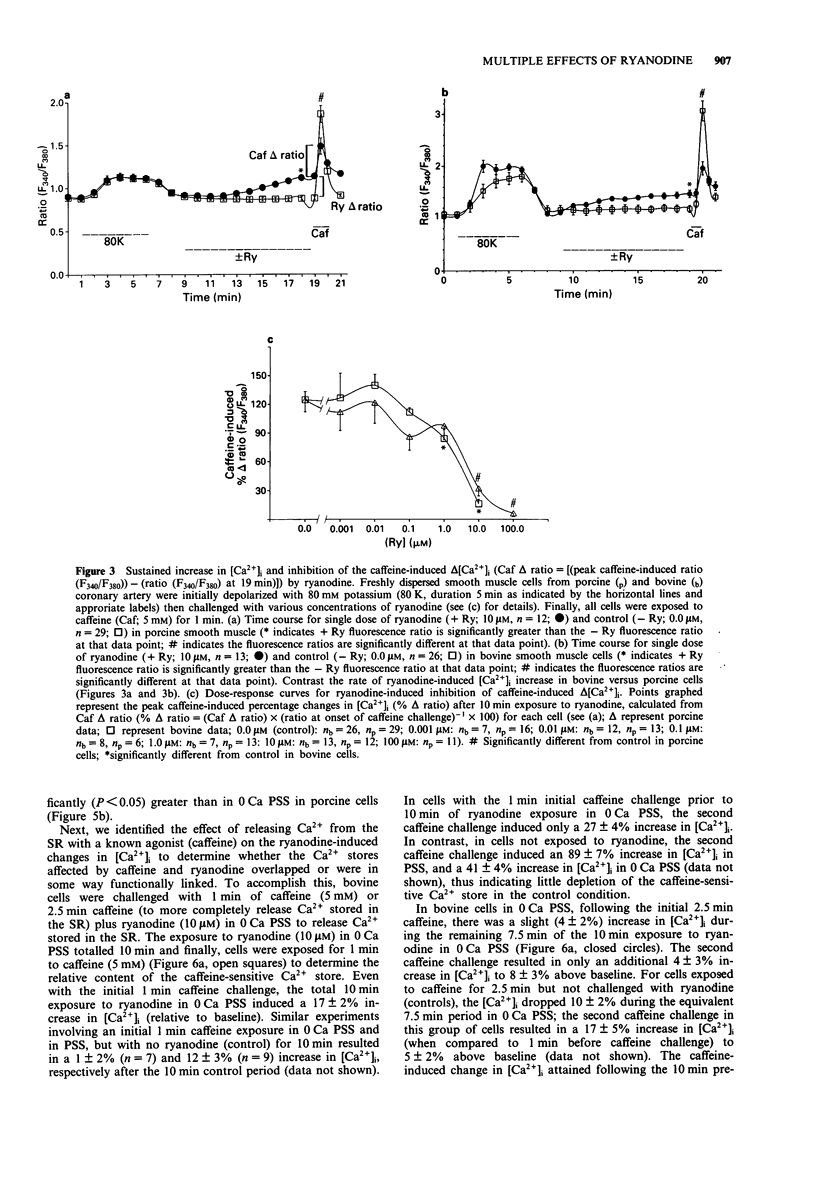
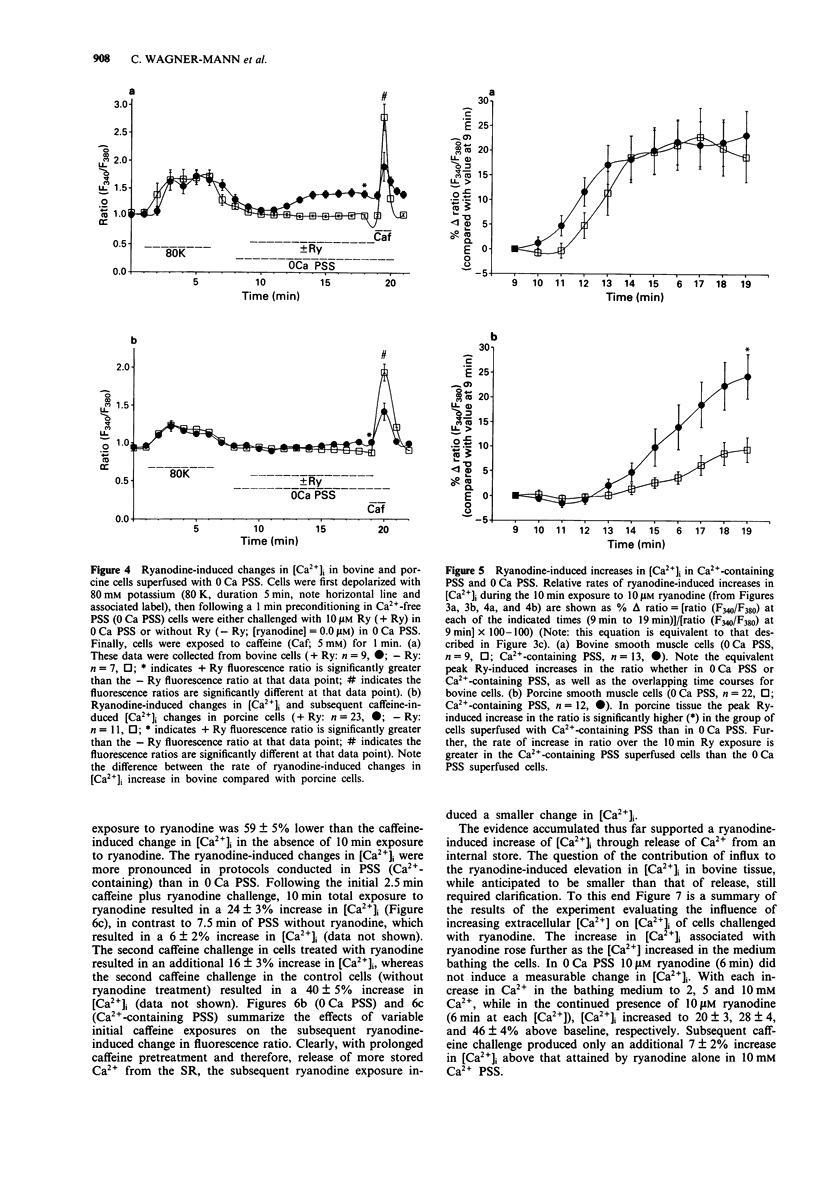
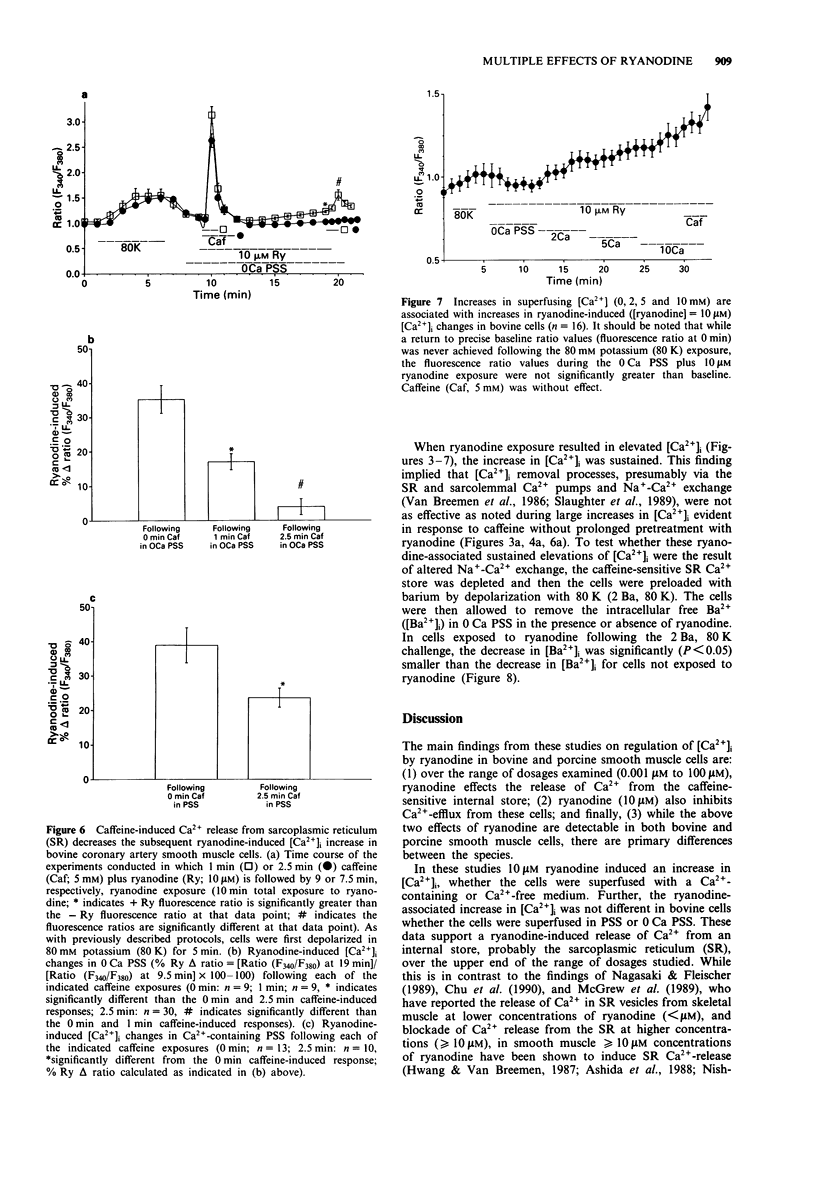
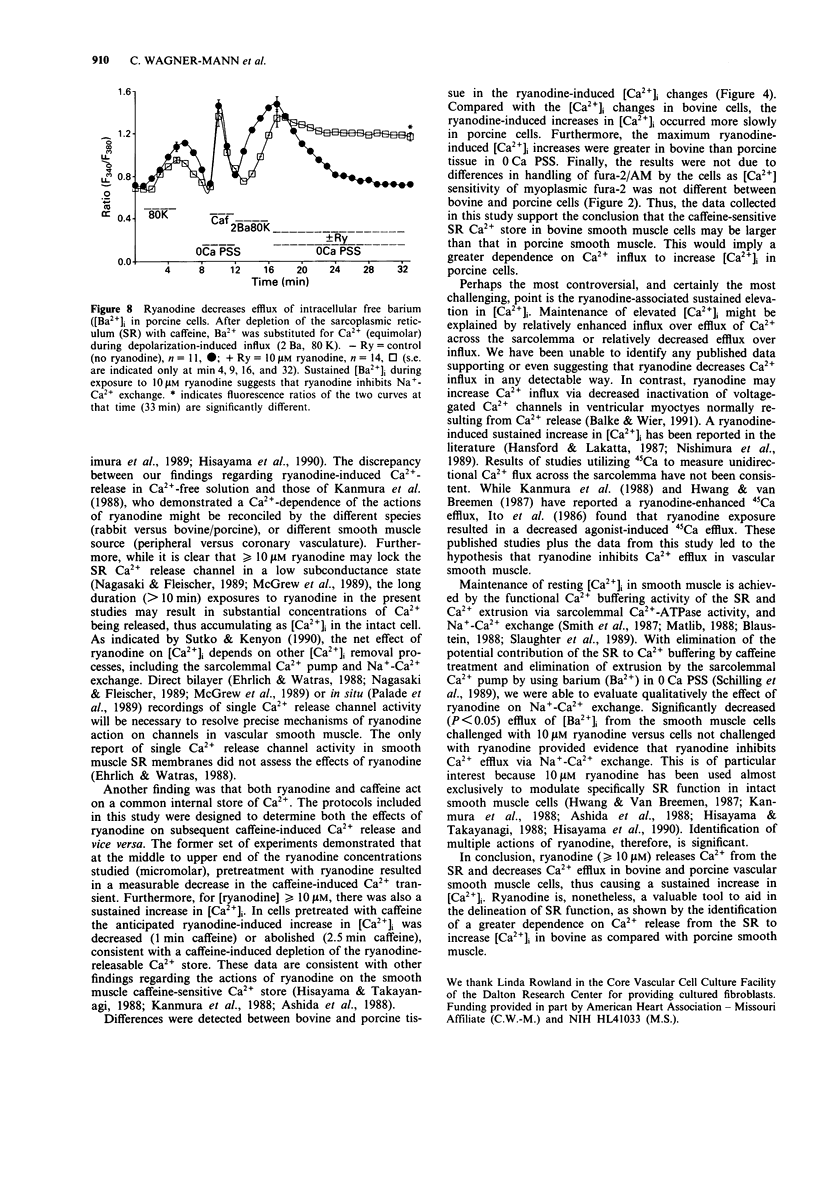

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Potassium, chloride and non-selective cation conductances opened by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Apr;423:551–568. doi: 10.1113/jphysiol.1990.sp018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida T., Schaeffer J., Goldman W. F., Wade J. B., Blaustein M. P. Role of sarcoplasmic reticulum in arterial contraction: comparison of ryanodines's effect in a conduit and a muscular artery. Circ Res. 1988 Apr;62(4):854–863. doi: 10.1161/01.res.62.4.854. [DOI] [PubMed] [Google Scholar]
- Balke C. W., Wier W. G. Ryanodine does not affect calcium current in guinea pig ventricular myocytes in which Ca2+ is buffered. Circ Res. 1991 Mar;68(3):897–902. doi: 10.1161/01.res.68.3.897. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Sodium/calcium exchange and the control of contractility in cardiac muscle and vascular smooth muscle. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S56–S68. [PubMed] [Google Scholar]
- Chu A., Díaz-Muñoz M., Hawkes M. J., Brush K., Hamilton S. L. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol Pharmacol. 1990 May;37(5):735–741. [PubMed] [Google Scholar]
- DeFeo T. T., Morgan K. G. Responses of enzymatically isolated mammalian vascular smooth muscle cells to pharmacological and electrical stimuli. Pflugers Arch. 1985 May;404(1):100–102. doi: 10.1007/BF00581502. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I., Okamoto Y. Ryanodine reveals multiple contractile and relaxant mechanisms in vascular smooth muscle: simultaneous measurements of mechanical activity and of cytoplasmic free Ca2+ level with fura-2. Br J Pharmacol. 1990 Aug;100(4):677–684. doi: 10.1111/j.1476-5381.1990.tb14075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I. Ryanodine: its possible mechanism of action in the caffeine-sensitive calcium store of smooth muscle. Pflugers Arch. 1988 Sep;412(4):376–381. doi: 10.1007/BF01907555. [DOI] [PubMed] [Google Scholar]
- Hwang K. S., van Breemen C. Ryanodine modulation of 45Ca efflux and tension in rabbit aortic smooth muscle. Pflugers Arch. 1987 Apr;408(4):343–350. doi: 10.1007/BF00581127. [DOI] [PubMed] [Google Scholar]
- Ito K., Takakura S., Sato K., Sutko J. L. Ryanodine inhibits the release of calcium from intracellular stores in guinea pig aortic smooth muscle. Circ Res. 1986 May;58(5):730–734. doi: 10.1161/01.res.58.5.730. [DOI] [PubMed] [Google Scholar]
- Jenden D. J., Fairhurst A. S. The pharmacology of ryanodine. Pharmacol Rev. 1969 Mar;21(1):1–25. [PubMed] [Google Scholar]
- Kanmura Y., Missiaen L., Raeymaekers L., Casteels R. Ryanodine reduces the amount of calcium in intracellular stores of smooth-muscle cells of the rabbit ear artery. Pflugers Arch. 1988 Dec;413(2):153–159. doi: 10.1007/BF00582525. [DOI] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb A. L., Owens G. K., Peach M. J. Evidence for endothelium-derived relaxing factor in cultured cells. Hypertension. 1985 Sep-Oct;7(5):804–807. doi: 10.1161/01.hyp.7.5.804. [DOI] [PubMed] [Google Scholar]
- Matlib M. A. Na+-Ca2+ exchange in sarcolemmal membrane vesicles of dog mesenteric artery. Am J Physiol. 1988 Sep;255(3 Pt 1):C323–C330. doi: 10.1152/ajpcell.1988.255.3.C323. [DOI] [PubMed] [Google Scholar]
- McGrew S. G., Wolleben C., Siegl P., Inui M., Fleischer S. Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry. 1989 Feb 21;28(4):1686–1691. doi: 10.1021/bi00430a039. [DOI] [PubMed] [Google Scholar]
- Nagasaki K., Fleischer S. Modulation of the calcium release channel of sarcoplasmic reticulum by adriamycin and other drugs. Cell Calcium. 1989 Jan;10(1):63–70. doi: 10.1016/0143-4160(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Khalil R. A., van Breemen C. Agonist-induced vascular tone. Hypertension. 1989 Jun;13(6 Pt 2):835–844. doi: 10.1161/01.hyp.13.6.835. [DOI] [PubMed] [Google Scholar]
- Palade P., Dettbarn C., Brunder D., Stein P., Hals G. Pharmacology of calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989 Apr;21(2):295–320. doi: 10.1007/BF00812074. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Slaughter R. S., Shevell J. L., Felix J. P., Garcia M. L., Kaczorowski G. J. High levels of sodium-calcium exchange in vascular smooth muscle sarcolemmal membrane vesicles. Biochemistry. 1989 May 2;28(9):3995–4002. doi: 10.1021/bi00435a055. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Cragoe E. J., Jr, Smith L. Na+/Ca2+ antiport in cultured arterial smooth muscle cells. Inhibition by magnesium and other divalent cations. J Biol Chem. 1987 Sep 5;262(25):11988–11994. [PubMed] [Google Scholar]
- Sutko J. L., Ito K., Kenyon J. L. Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. Fed Proc. 1985 Dec;44(15):2984–2988. [PubMed] [Google Scholar]
- Sutko J. L., Kenyon J. L. Actions of ryanodine. J Gen Physiol. 1990 Aug;96(2):439–445. doi: 10.1085/jgp.96.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Sturek M., Miller R. J. Measurement of neuronal Ca2+ transients using simultaneous microfluorimetry and electrophysiology. Pflugers Arch. 1988 Jul;412(1-2):216–223. doi: 10.1007/BF00583753. [DOI] [PubMed] [Google Scholar]
- Van Dijk A. M., Laird J. D. Characterization of single isolated vascular smooth muscle cells from bovine coronary artery. Blood Vessels. 1984;21(6):267–278. doi: 10.1159/000158529. [DOI] [PubMed] [Google Scholar]
- Wagner-Mann C., Bowman L., Sturek M. Primary action of endothelin on Ca release in bovine coronary artery smooth muscle cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C763–C770. doi: 10.1152/ajpcell.1991.260.4.C763. [DOI] [PubMed] [Google Scholar]
- Wagner-Mann C., Sturek M. Endothelin mediates Ca influx and release in porcine coronary smooth muscle cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C771–C777. doi: 10.1152/ajpcell.1991.260.4.C771. [DOI] [PubMed] [Google Scholar]
- Warshaw D. M., Szarek J. L., Hubbard M. S., Evans J. N. Pharmacology and force development of single freshly isolated bovine carotid artery smooth muscle cells. Circ Res. 1986 Mar;58(3):399–406. doi: 10.1161/01.res.58.3.399. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K., Yamamoto H., Hwang K., Twort C. Vascular smooth muscle sarcoplasmic reticulum. Function and mechanisms of Ca2+ release. Ann N Y Acad Sci. 1988;522:60–73. doi: 10.1111/j.1749-6632.1988.tb33344.x. [DOI] [PubMed] [Google Scholar]



