Abstract
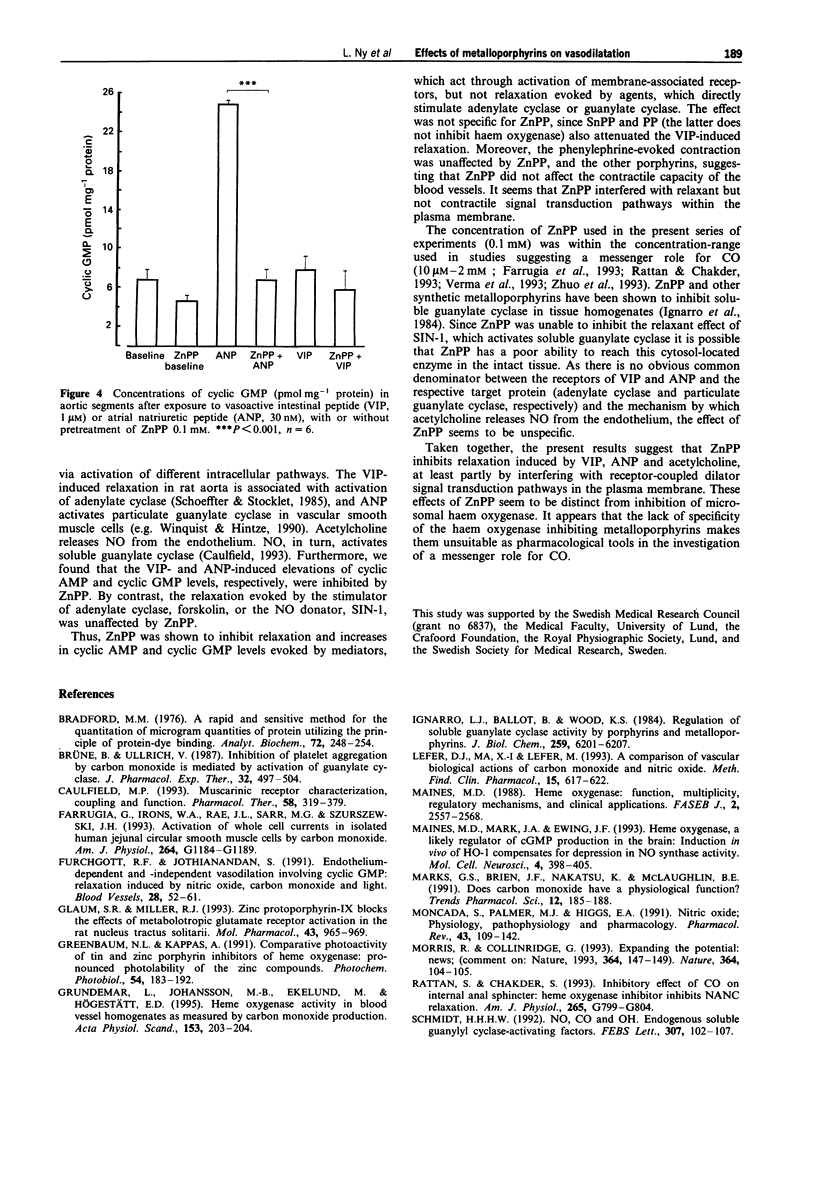
1. Carbon monoxide (CO), produced by haem oxygenase through degradation of haem, has been claimed to be a neuromessenger and a possible regulator of vascular tone. We examined whether the haem oxygenase inhibitor, zinc protoporphyrin-IX (ZnPP) and other porphyrins affect the relaxation evoked by various agents in the rat isolated aorta. 2. Pretreatment with ZnPP (0.1 mM) virtually abolished the relaxation evoked by vasoactive intestinal peptide (VIP) and atrial natriuretic peptide (ANP). ZnPP also evoked a rightward shift of the concentration-response curve for the relaxation induced by acetylcholine. 3. In contrast, ZnPP did not affect the relaxation evoked by forskolin and 3-morpholino-sydnonimine, agents which directly activate adenylate and guanylate cyclase, respectively. 4. Although, less effective than ZnPP, tin protoporphyrin-IX (SnPP; 0.1 mM) and protoporphyrin-IX (PP; 0.1 mM) also attenuated the VIP-evoked relaxation. 5. The elevation of cyclic AMP and cyclic GMP levels evoked by VIP and ANP, respectively, were abolished by pretreatment with ZnPP (0.1 mM). 6. ZnPP, SnPP and PP did not affect the contraction evoked by phenylephrine. 7. The results show that ZnPP inhibits relaxation induced by VIP, ANP and acetylcholine, probably by interfering with membrane receptor-coupled signal transduction pathways. This inhibition does not seem to be dependent upon inhibition of haem oxygenase. The lack of specificity of the haem oxygenase inhibiting metalloporphyrins makes them less suitable as pharmacological tools in the investigation of a messenger role for CO.
Full text
PDF

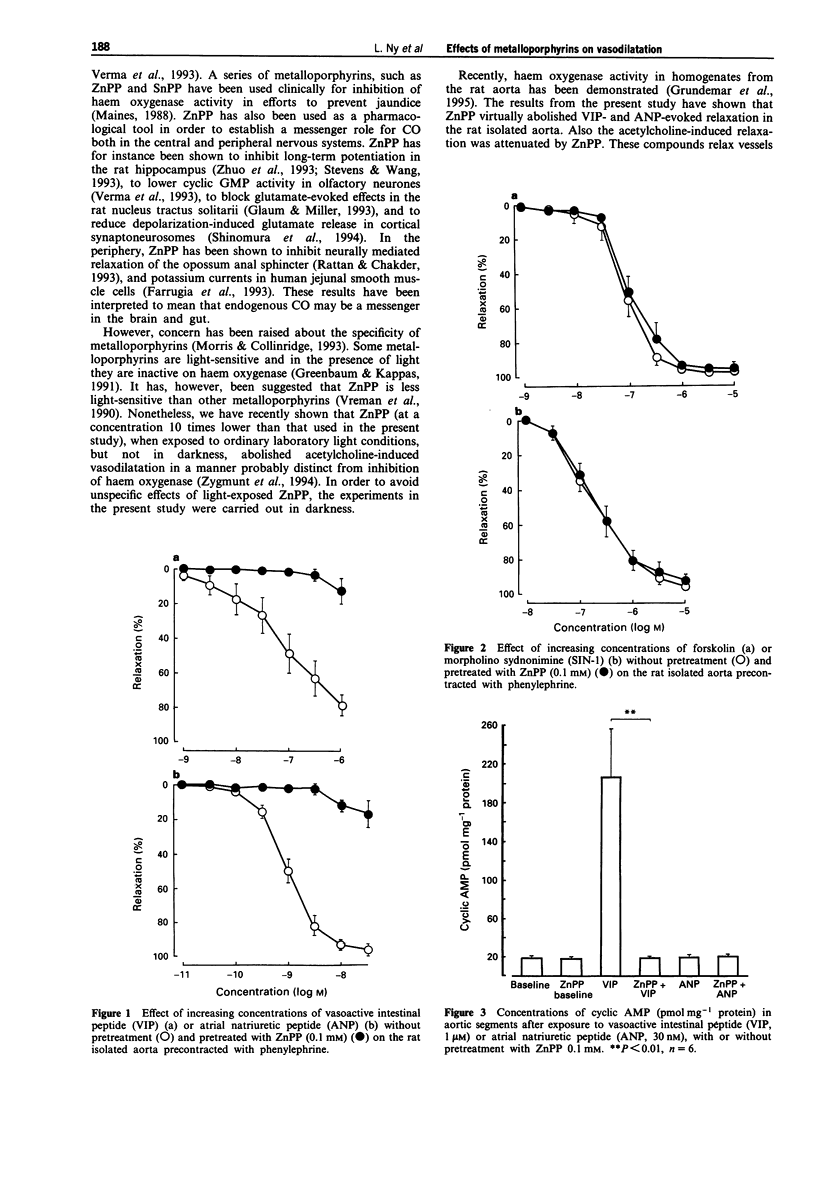

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987 Oct;32(4):497–504. [PubMed] [Google Scholar]
- Caulfield M. P. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993 Jun;58(3):319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Farrugia G., Irons W. A., Rae J. L., Sarr M. G., Szurszewski J. H. Activation of whole cell currents in isolated human jejunal circular smooth muscle cells by carbon monoxide. Am J Physiol. 1993 Jun;264(6 Pt 1):G1184–G1189. doi: 10.1152/ajpgi.1993.264.6.G1184. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28(1-3):52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- Glaum S. R., Miller R. J. Zinc protoporphyrin-IX blocks the effects of metabotropic glutamate receptor activation in the rat nucleus tractus solitarii. Mol Pharmacol. 1993 Jun;43(6):965–969. [PubMed] [Google Scholar]
- Greenbaum N. L., Kappas A. Comparative photoactivity of tin and zinc porphyrin inhibitors of heme oxygenase: pronounced photolability of the zinc compounds. Photochem Photobiol. 1991 Aug;54(2):183–192. doi: 10.1111/j.1751-1097.1991.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Grundemar L., Johansson M. B., Ekelund M., Högestätt E. D. Haem oxygenase activity in blood vessel homogenates as measured by carbon monoxide production. Acta Physiol Scand. 1995 Feb;153(2):203–204. doi: 10.1111/j.1748-1716.1995.tb09852.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Ballot B., Wood K. S. Regulation of soluble guanylate cyclase activity by porphyrins and metalloporphyrins. J Biol Chem. 1984 May 25;259(10):6201–6207. [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Marks G. S., Brien J. F., Nakatsu K., McLaughlin B. E. Does carbon monoxide have a physiological function? Trends Pharmacol Sci. 1991 May;12(5):185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morris R., Collingridge G. Neuroscience. Expanding the potential. Nature. 1993 Jul 8;364(6433):104–105. doi: 10.1038/364104a0. [DOI] [PubMed] [Google Scholar]
- Rattan S., Chakder S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993 Oct;265(4 Pt 1):G799–G804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H. NO., CO and .OH. Endogenous soluble guanylyl cyclase-activating factors. FEBS Lett. 1992 Jul 27;307(1):102–107. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Stoclet J. C. Effect of vasoactive intestinal polypeptide (VIP) on cyclic AMP level and relaxation in rat isolated aorta. Eur J Pharmacol. 1985 Feb 26;109(2):275–279. doi: 10.1016/0014-2999(85)90430-3. [DOI] [PubMed] [Google Scholar]
- Shinomura T., Nakao S., Mori K. Reduction of depolarization-induced glutamate release by heme oxygenase inhibitor: possible role of carbon monoxide in synaptic transmission. Neurosci Lett. 1994 Jan 31;166(2):131–134. doi: 10.1016/0304-3940(94)90468-5. [DOI] [PubMed] [Google Scholar]
- Stevens C. F., Wang Y. Reversal of long-term potentiation by inhibitors of haem oxygenase. Nature. 1993 Jul 8;364(6433):147–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Gillman M. J., Downum K. R., Stevenson D. K. In vitro generation of carbon monoxide from organic molecules and synthetic metalloporphyrins mediated by light. Dev Pharmacol Ther. 1990;15(2):112–124. doi: 10.1159/000457630. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Stevenson D. K. Heme oxygenase activity as measured by carbon monoxide production. Anal Biochem. 1988 Jan;168(1):31–38. doi: 10.1016/0003-2697(88)90006-1. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Hintze T. H. Mechanisms of atrial natriuretic factor-induced vasodilation. Pharmacol Ther. 1990;48(3):417–426. doi: 10.1016/0163-7258(90)90058-a. [DOI] [PubMed] [Google Scholar]
- Zhuo M., Small S. A., Kandel E. R., Hawkins R. D. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993 Jun 25;260(5116):1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. M., Högestätt E. D., Grundemar L. Light-dependent effects of zinc protoporphyrin IX on endothelium-dependent relaxation resistant to N omega-nitro-L-arginine. Acta Physiol Scand. 1994 Oct;152(2):137–143. doi: 10.1111/j.1748-1716.1994.tb09793.x. [DOI] [PubMed] [Google Scholar]


