Abstract
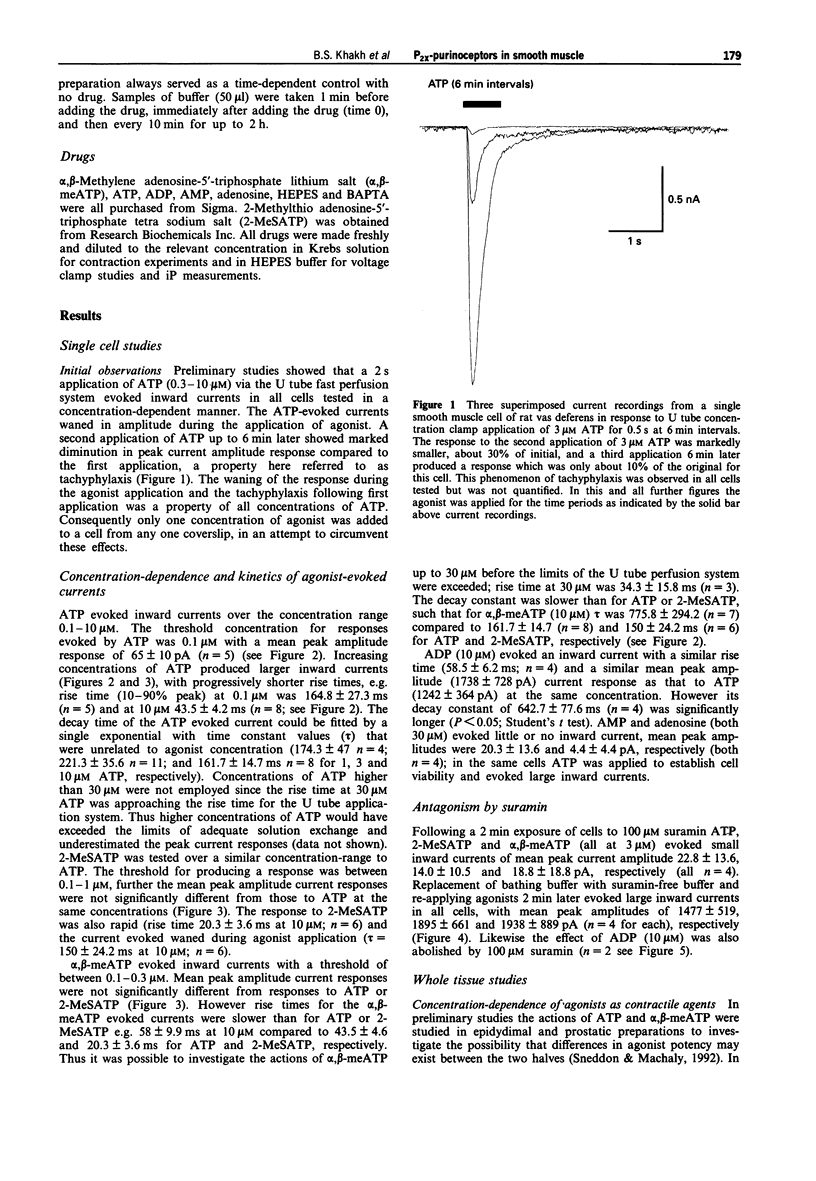
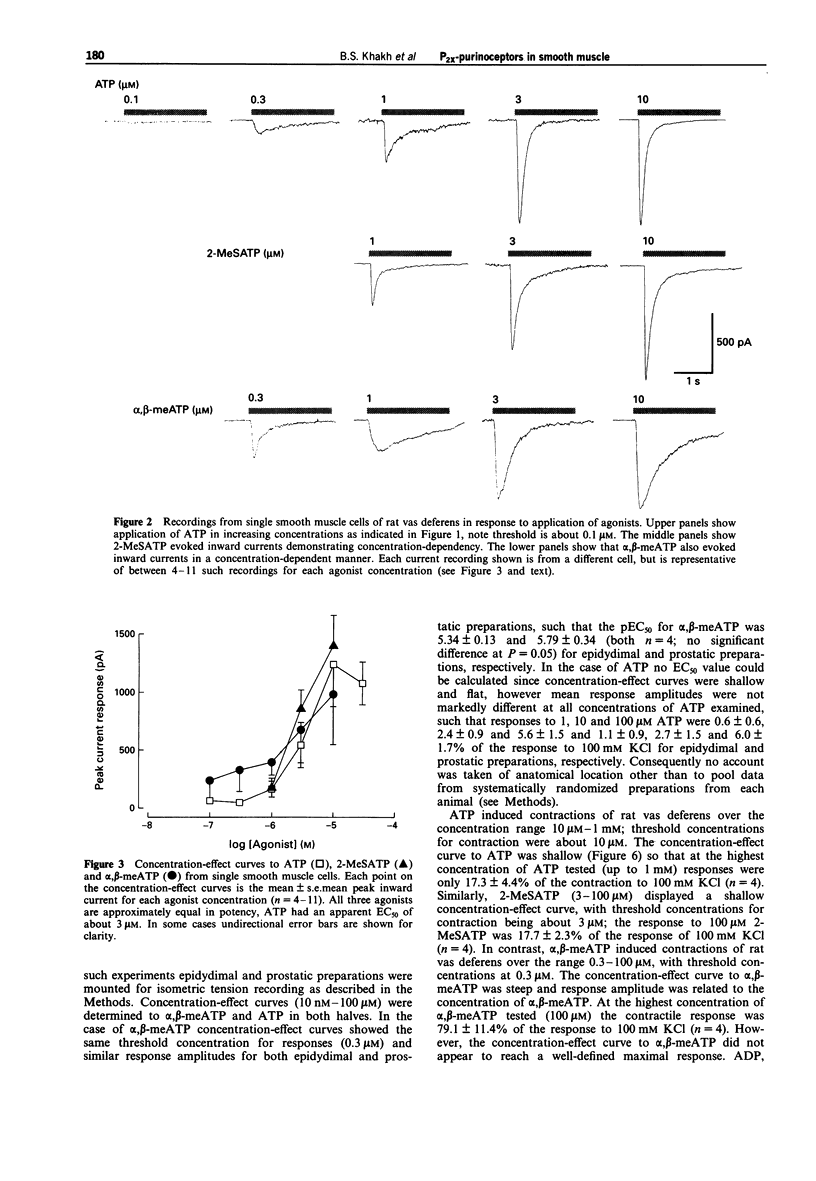
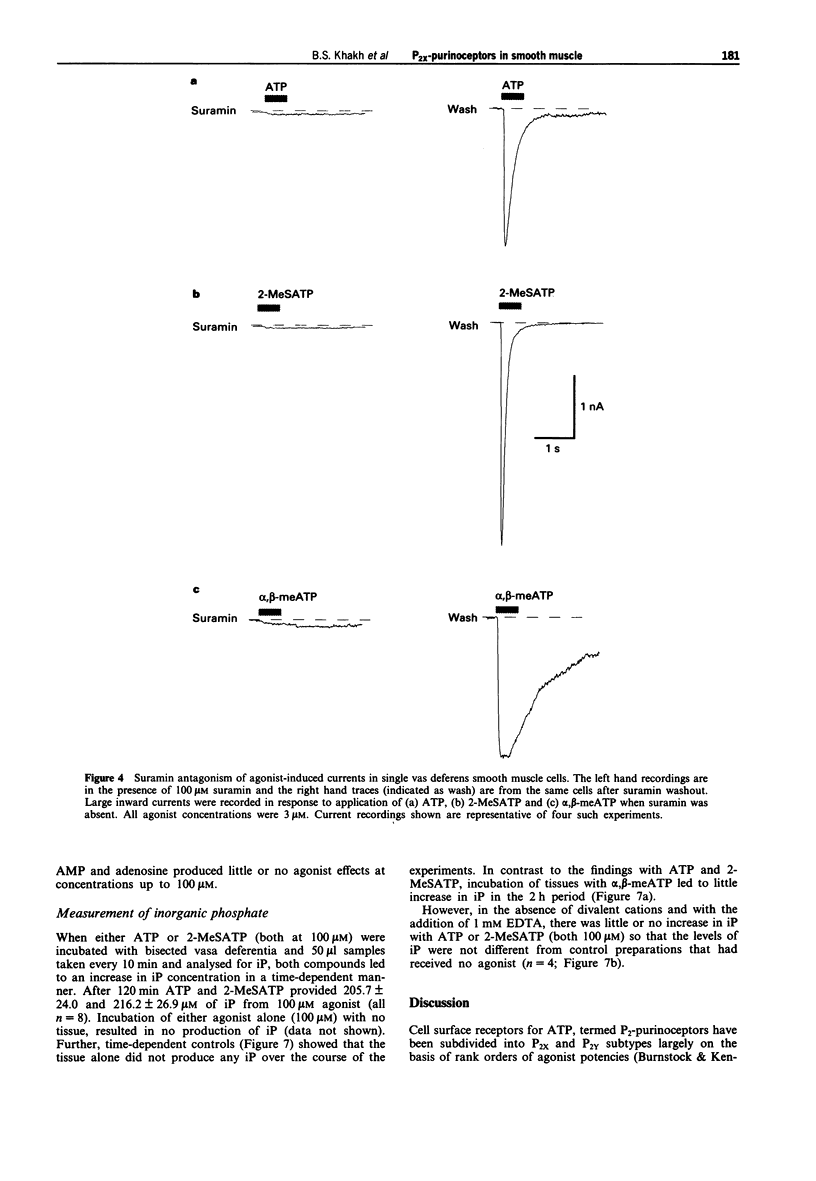
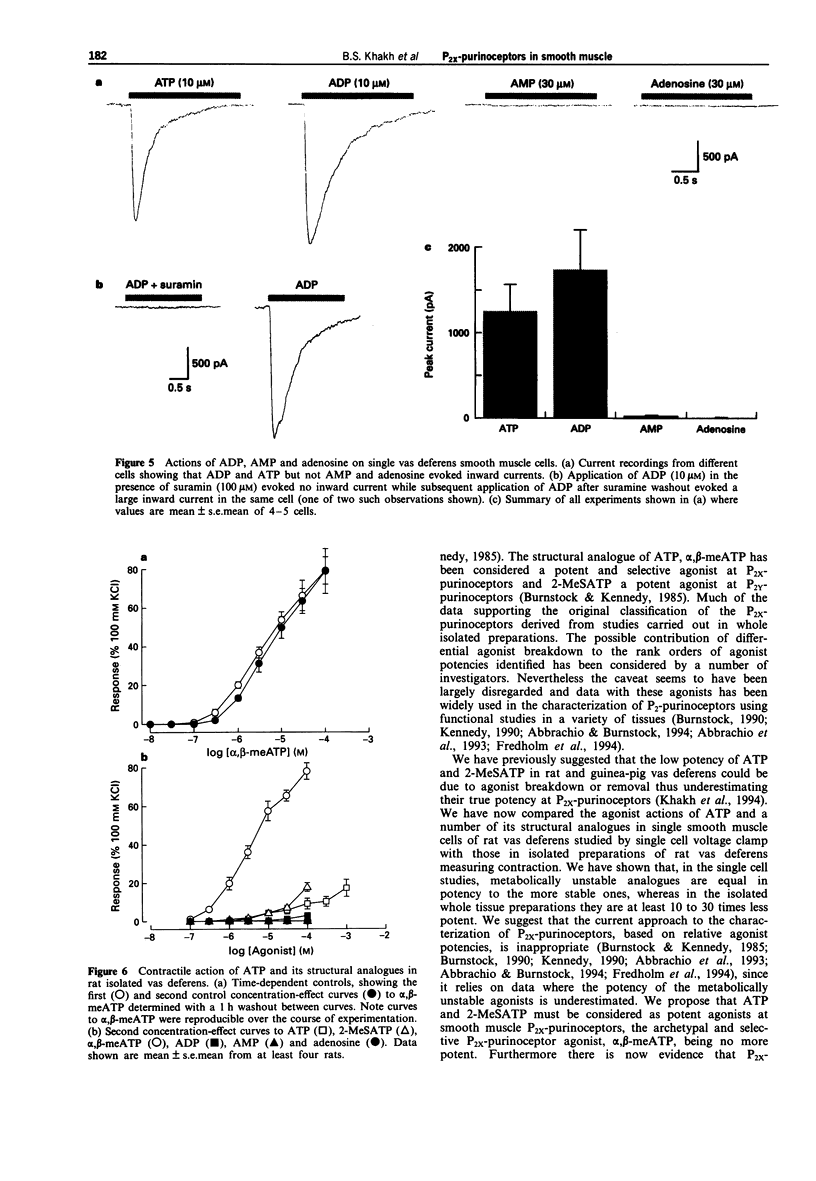
1. We have studied both the electrophysiological and contractile effects of the purine nucleotide, adenosine-5'-triphosphate (ATP), as well as a number of its structural analogues as agonists at P2X purinoceptors in the rat vas deferens in vitro. 2. Electrophysiological effects were investigated by a whole cell voltage clamp technique (holding potential-70 mV) with fast flow concentration-clamp applications of agonists in single isolated smooth muscle cells. ATP, 2-methylthio adenosine-5'-triphosphate (2-MeSATP) and alpha,beta methylene adenosine-5'-triphosphate (alpha,beta-meATP) all evoked inward currents over a similar concentration range (0.3-10 microM), being approximately equipotent with similar concentrations for threshold effects (0.3 microM). ADP (10 microM) also evoked a rapid current of similar peak amplitude to that seen with ATP (10 microM). 3. alpha,beta-meATP was the most potent agonist in producing concentrations of the rat vas deferens whole tissue preparation, with a threshold concentration equal to that in the electrophysiological studies (0.3 microM). However, ATP and 2-MeSATP were at least ten times less potent in studies measuring contraction than in the electrophysiological studies. Furthermore, their concentration-effect curves were shallow with smaller maximal responses than could be achieved with alpha,beta-meATP. ADP, AMP and adenosine were inactive at concentrations up to 1 mM. The rank order of agonist potencies observed for contraction was alpha,beta-meATP >> ATP = 2-MeSATP.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbracchio M. P., Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Amobi N. I., Smith I. C. Adrenergic and 'non-adrenergic' contributions to the two-component tetanus in the rat vas deferens. Eur J Pharmacol. 1987 Mar 17;135(2):173–182. doi: 10.1016/0014-2999(87)90609-1. [DOI] [PubMed] [Google Scholar]
- Amobi N., Smith I. C. Effects of alpha,beta-methylene ATP on biphasic responses of rat vas deferens. Eur J Pharmacol. 1987 Jan 6;133(1):75–82. doi: 10.1016/0014-2999(87)90207-x. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The changing face of autonomic neurotransmission. Acta Physiol Scand. 1986 Jan;126(1):67–91. doi: 10.1111/j.1748-1716.1986.tb07790.x. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991 Sep;76(5):677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Kennedy C. Characterization of P2-purinoceptors in the smooth muscle of the rat tail artery: a comparison between contractile and electrophysiological responses. Br J Pharmacol. 1994 Nov;113(3):853–860. doi: 10.1111/j.1476-5381.1994.tb17071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- French A. M., Scott N. C. Evidence to support the hypothesis that ATP is a co-transmitter in rat vas deferens. Experientia. 1983 Mar 15;39(3):264–266. doi: 10.1007/BF01955295. [DOI] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess H. H., Derr J. E. Assay of inorganic and organic phosphorus in the 0.1-5 nanomole range. Anal Biochem. 1975 Feb;63(2):607–613. doi: 10.1016/0003-2697(75)90388-7. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N. Adenosine triphosphate-activated inward current in isolated smooth muscle cells from rat vas deferens. Pflugers Arch. 1987 Aug;409(6):644–646. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Machaly M. Regional variation in purinergic and adrenergic responses in isolated vas deferens of rat, rabbit and guinea-pig. J Auton Pharmacol. 1992 Dec;12(6):421–428. doi: 10.1111/j.1474-8673.1992.tb00390.x. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Discrete events measure single quanta of adenosine 5'-triphosphate secreted from sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1984 Sep;13(1):21–28. doi: 10.1016/0306-4522(84)90256-2. [DOI] [PubMed] [Google Scholar]
- Taylor D. A., Wiese S., Faison E. P., Yarbrough G. G. Pharmacological characterization of purinergic receptors in the rat vas deferens. J Pharmacol Exp Ther. 1983 Jan;224(1):40–45. [PubMed] [Google Scholar]
- Trezise D. J., Bell N. J., Kennedy I., Humphrey P. P. Effects of divalent cations on the potency of ATP and related agonists in the rat isolated vagus nerve: implications for P2 purinoceptor classification. Br J Pharmacol. 1994 Oct;113(2):463–470. doi: 10.1111/j.1476-5381.1994.tb17012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994 Oct 6;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. ATP analogues and the guinea-pig taenia coli: a comparison of the structure-activity relationships of ectonucleotidases with those of the P2-purinoceptor. Eur J Pharmacol. 1986 Oct 7;129(3):217–224. doi: 10.1016/0014-2999(86)90431-0. [DOI] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. The structure-activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: evidence that dephosphorylation of ATP analogues reduces pharmacological potency. Eur J Pharmacol. 1987 Sep 2;141(1):123–130. doi: 10.1016/0014-2999(87)90418-3. [DOI] [PubMed] [Google Scholar]
- Ziganshin A. U., Ziganshina L. E., King B. E., Burnstock G. Characteristics of ecto-ATPase of Xenopus oocytes and the inhibitory actions of suramin on ATP breakdown. Pflugers Arch. 1995 Jan;429(3):412–418. doi: 10.1007/BF00374157. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994 Oct;17(10):420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol Sci. 1991 Sep;12(9):319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]


