Abstract
1. Many endothelium-dependent vasodilators hyperpolarize the endothelial cells in blood vessels. It is not known whether these hyperpolarizations are linked to nitric oxide synthesis or to an endothelium-derived hyperpolarizing phenomenon, since most of the vasodilators release both factors. In this context, we first verified that the endothelium-dependent relaxations induced by 5-hydroxytryptamine (5-HT) on pig coronary arteries are due only to the activation of the nitric oxide pathway. Then we studied the effects of 5-HT on membrane potential of endothelial and smooth muscle cells. 2. In the absence of endothelium, 5-HT caused a concentration-dependent contraction of coronary artery strips. No change of the smooth muscle cell membrane potential was observed during contraction to 1 microM 5-HT. 3. In the presence of 1 microM ketanserin to suppress the contractile effect of 5-HT, 5-HT induced concentration-dependent relaxation of endothelium-intact strips precontracted by 10 microM prostaglandin F2 alpha (PGF2 alpha). These relaxations were suppressed by 1 microM NG-nitro-L-arginine, an inhibitor of nitric oxide synthesis, showing that they were produced predominantly by nitric oxide. 4. In the presence of 1 microM ketanserin, 1 microM 5-HT did not change the smooth muscle cell membrane potential of strips precontracted by either 10 microM PGF2 alpha or by 10 microM acetylcholine (ACh). In the same conditions, 1 microM 5-HT caused a weak 2.6 +/- 0.4 mV hyperpolarization, of the endothelial cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

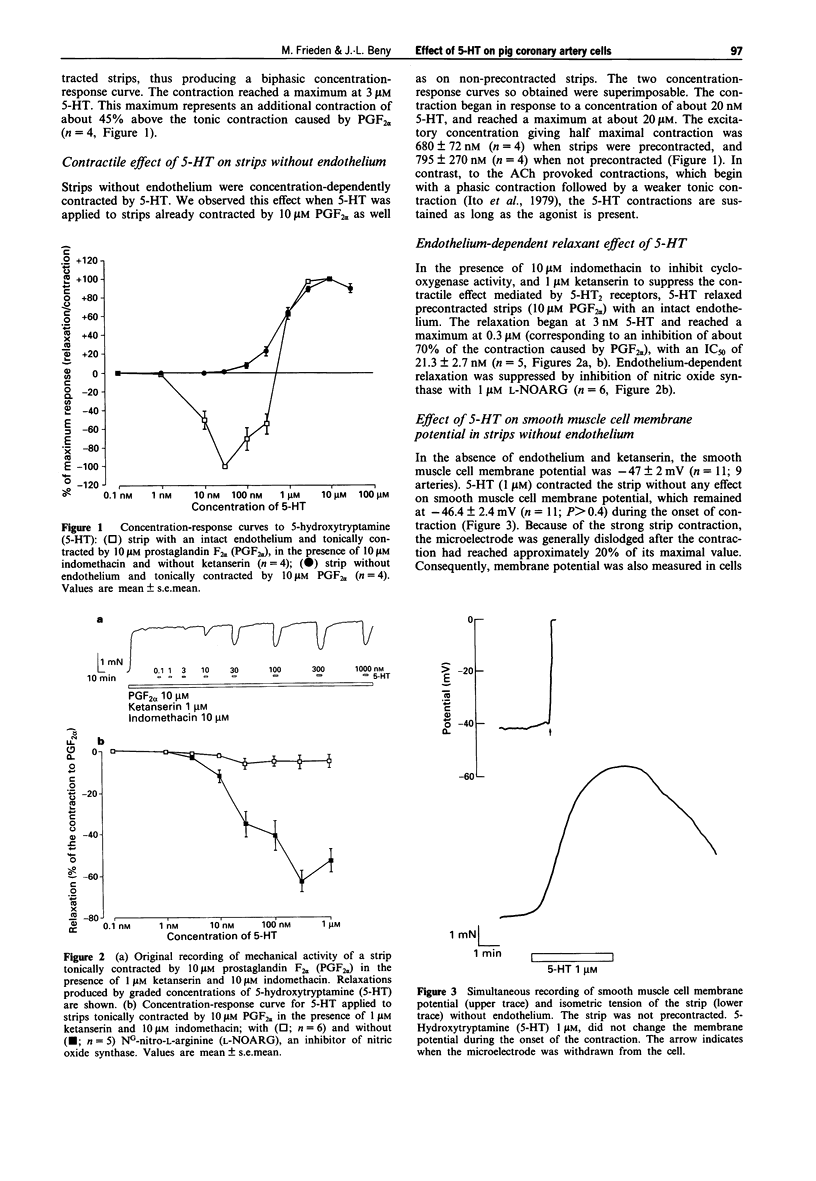
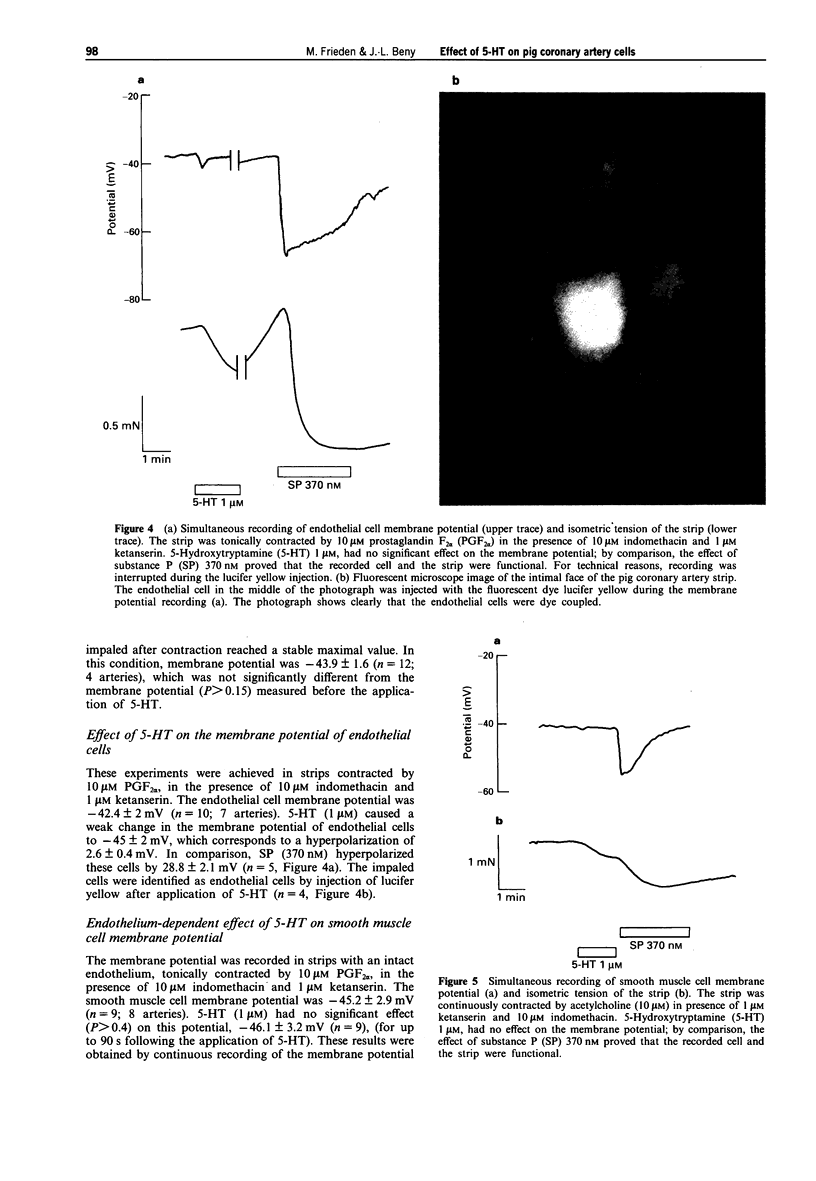


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A. 5-HT receptors in the coronary circulation. Trends Pharmacol Sci. 1989 Mar;10(3):89–90. doi: 10.1016/0165-6147(89)90197-1. [DOI] [PubMed] [Google Scholar]
- Bax W. A., Renzenbrink G. J., Van Heuven-Nolsen D., Thijssen E. J., Bos E., Saxena P. R. 5-HT receptors mediating contractions of the isolated human coronary artery. Eur J Pharmacol. 1993 Aug 3;239(1-3):203–210. doi: 10.1016/0014-2999(93)90995-t. [DOI] [PubMed] [Google Scholar]
- Beny J. L., Brunet P. C., Huggel H. Effect of mechanical stimulation, substance P and vasoactive intestinal polypeptide on the electrical and mechanical activities of circular smooth muscles from pig coronary arteries contracted with acetylcholine: role of endothelium. Pharmacology. 1986;33(2):61–68. doi: 10.1159/000138202. [DOI] [PubMed] [Google Scholar]
- Beny J. L., Brunet P., Huggel H. Interaction of bradykinin and des-Arg9-bradykinin with isolated pig coronary arteries: mechanical and electrophysiological events. Regul Pept. 1987 Apr;17(4):181–190. doi: 10.1016/0167-0115(87)90061-9. [DOI] [PubMed] [Google Scholar]
- Brunet P. C., Bény J. L. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26(4):228–234. doi: 10.1159/000158770. [DOI] [PubMed] [Google Scholar]
- Bruning T. A., Chang P. C., Blauw G. J., Vermeij P., van Zwieten P. A. Serotonin-induced vasodilatation in the human forearm is mediated by the "nitric oxide-pathway": no evidence for involvement of the 5-HT3-receptor. J Cardiovasc Pharmacol. 1993 Jul;22(1):44–51. doi: 10.1097/00005344-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C. Neither nitric oxide nor nitroglycerin accounts for all the characteristics of endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988;25(6):308–311. [PubMed] [Google Scholar]
- Bény J. L., Connat J. L. An electron-microscopic study of smooth muscle cell dye coupling in the pig coronary arteries. Role of gap junctions. Circ Res. 1992 Jan;70(1):49–55. doi: 10.1161/01.res.70.1.49. [DOI] [PubMed] [Google Scholar]
- Bény J. L. Effect of substance P on the membrane potential of coronary arterial endothelial cells in situ. Agents Actions. 1990 Nov;31(3-4):317–320. doi: 10.1007/BF01997626. [DOI] [PubMed] [Google Scholar]
- Bény J. L. Endothelial and smooth muscle cells hyperpolarized by bradykinin are not dye coupled. Am J Physiol. 1990 Mar;258(3 Pt 2):H836–H841. doi: 10.1152/ajpheart.1990.258.3.H836. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Gribi F. Dye and electrical coupling of endothelial cells in situ. Tissue Cell. 1989;21(6):797–802. doi: 10.1016/0040-8166(89)90030-x. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol. 1994 Apr;266(4 Pt 2):H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983 Oct 13;305(5935):627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Shepherd J. T., Vanhoutte P. M. 5-Hydroxytryptamine can mediate endothelium-dependent relaxation of coronary arteries. Am J Physiol. 1983 Dec;245(6):H1077–H1080. doi: 10.1152/ajpheart.1983.245.6.H1077. [DOI] [PubMed] [Google Scholar]
- Garland C. J. The role of membrane depolarization in the contractile response of the rabbit basilar artery to 5-hydroxytryptamine. J Physiol. 1987 Nov;392:333–348. doi: 10.1113/jphysiol.1987.sp016783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. S., Vanhoutte P. M. Serotonin and the vascular system. Role in health and disease, and implications for therapy. Drugs. 1986 Feb;31(2):149–163. doi: 10.2165/00003495-198631020-00004. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J Physiol. 1979 Sep;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T. Pharmacomechanical coupling in vascular smooth muscle cells--an overview. Jpn J Pharmacol. 1991 Jan;55(1):1–9. doi: 10.1254/jjp.55.1. [DOI] [PubMed] [Google Scholar]
- Kilpatrick E. V., Cocks T. M. Evidence for differential roles of nitric oxide (NO) and hyperpolarization in endothelium-dependent relaxation of pig isolated coronary artery. Br J Pharmacol. 1994 Jun;112(2):557–565. doi: 10.1111/j.1476-5381.1994.tb13110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Awouters F., Kennis L., Laduron P. M., Vandenberk J., Janssen P. A. Receptor binding profile of R 41 468, a novel antagonist at 5-HT2 receptors. Life Sci. 1981 Mar 2;28(9):1015–1022. doi: 10.1016/0024-3205(81)90747-5. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Molderings G. J., Engel G., Roth E., Göthert M. Characterization of an endothelial 5-hydroxytryptamine (5-HT) receptor mediating relaxation of the porcine coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1989 Sep;340(3):300–308. doi: 10.1007/BF00168514. [DOI] [PubMed] [Google Scholar]
- Pacicca C., von der Weid P. Y., Beny J. L. Effect of nitro-L-arginine on endothelium-dependent hyperpolarizations and relaxations of pig coronary arteries. J Physiol. 1992 Nov;457:247–256. doi: 10.1113/jphysiol.1992.sp019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratz P. H., Flaim S. F. Acetylcholine- and 5-hydroxytryptamine-stimulated contraction and calcium uptake in bovine coronary arteries: evidence for two populations of receptor-operated calcium channels. J Pharmacol Exp Ther. 1985 Sep;234(3):641–647. [PubMed] [Google Scholar]
- Richard V., Tanner F. C., Tschudi M., Lüscher T. F. Different activation of L-arginine pathway by bradykinin, serotonin, and clonidine in coronary arteries. Am J Physiol. 1990 Nov;259(5 Pt 2):H1433–H1439. doi: 10.1152/ajpheart.1990.259.5.H1433. [DOI] [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Hoyer D. 5-Hydroxytryptamine (5-HT)-induced endothelium-dependent relaxation of pig coronary arteries is mediated by 5-HT receptors similar to the 5-HT1D receptor subtype. J Pharmacol Exp Ther. 1990 Jan;252(1):387–395. [PubMed] [Google Scholar]
- Van Nueten J. M., Janssen P. A., Van Beek J., Xhonneux R., Verbeuren T. J., Vanhoutte P. M. Vascular effects of ketanserin (R 41 468), a novel antagonist of 5-HT2 serotonergic receptors. J Pharmacol Exp Ther. 1981 Jul;218(1):217–230. [PubMed] [Google Scholar]
- Vanhoutte P. M. Cardiovascular effects of serotonin. J Cardiovasc Pharmacol. 1987;10 (Suppl 3):S8–11. [PubMed] [Google Scholar]
- Vanhoutte P. M., Cohen R. A., Van Nueten J. M. Serotonin and arterial vessels. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S421–S428. doi: 10.1097/00005344-198406002-00017. [DOI] [PubMed] [Google Scholar]
- von der Weid P. Y., Bény J. L. Simultaneous oscillations in the membrane potential of pig coronary artery endothelial and smooth muscle cells. J Physiol. 1993 Nov;471:13–24. doi: 10.1113/jphysiol.1993.sp019888. [DOI] [PMC free article] [PubMed] [Google Scholar]



