Abstract
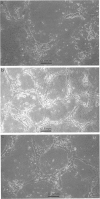
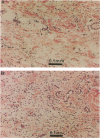
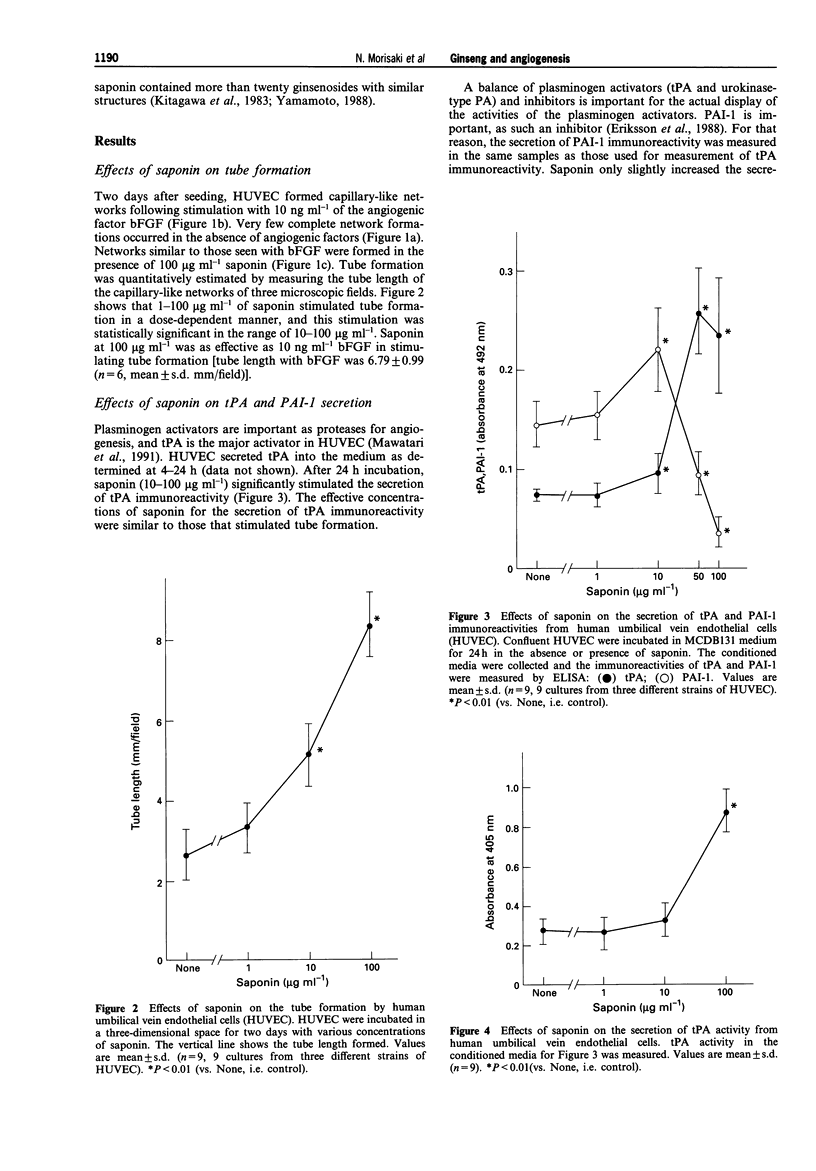
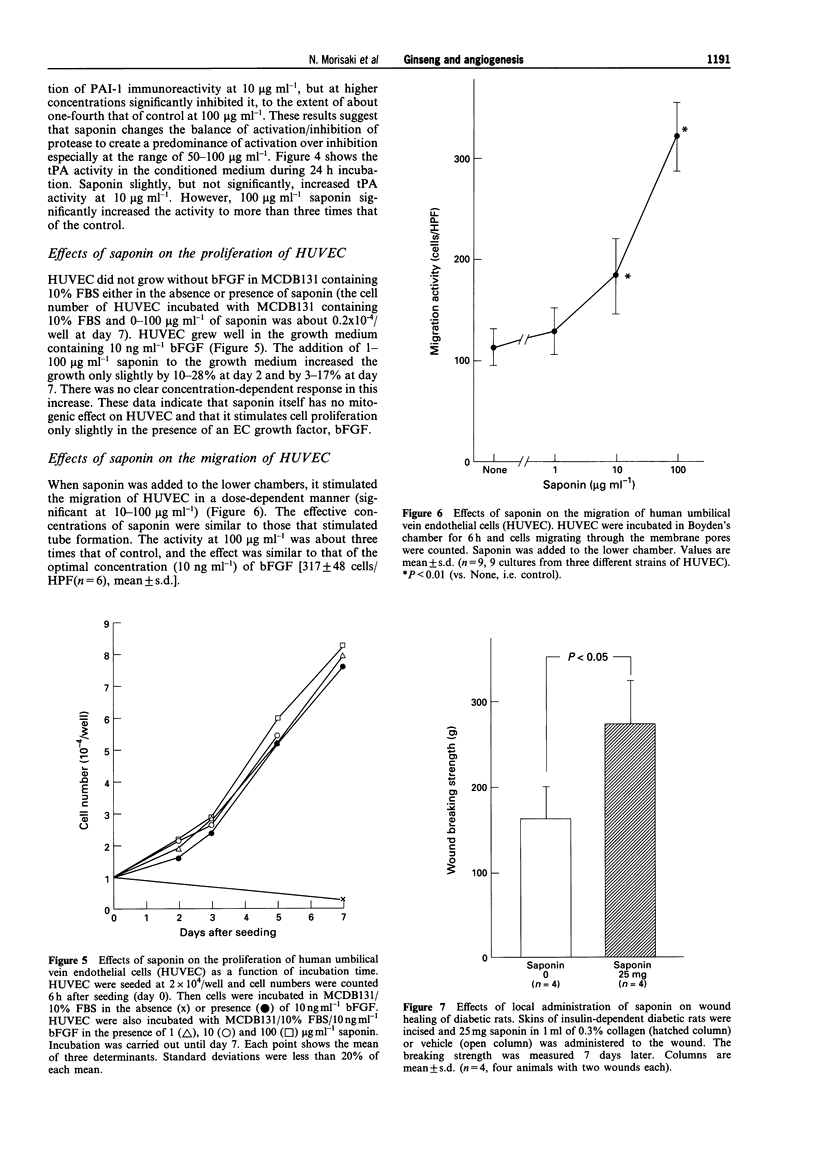
1. The effects of saponin from Ginseng Radix rubra on angiogenesis (tube formation) and its key steps (protease secretion, proliferation and migration) in human umbilical vein endothelial cells (HUVEC) were examined to elucidate the mechanism of the tissue repairing effects of Ginseng Radix rubra. The effect on a wound healing model was also studied. 2. Tube formation was measured by an in vitro system. The activity and immunoreactivity of tissue-type plasminogen activator (tPA) as a protease for angiogenesis and the immunoreactivity of its inhibitor, plasminogen activator inhibitor-1 (PAI-1), were measured in conditioned medium of HUVEC stimulated for 24 h with saponin. Cell proliferation was measured by counting the cell numbers at 2-7 days after seeding. Migration was measured by Boyden's chamber method. The effect on wound healing was studied in the skin of diabetic rats. 3. Saponin at 10-100 micrograms ml-1 significantly stimulated tube formation by HUVEC in a dose-dependent manner. Saponin in a similar concentration-range increased the secretion of tPA from HUVEC as estimated by immunoreactivity and enzyme activity. On the other hand, PAI-1 immunoreactivity was slightly increased at 10 micrograms ml-1 of saponin, but then was significantly decreased at 50 and 100 micrograms ml-1. Cell proliferation was only slightly enhanced by 1-100 micrograms ml-1 of saponin, but migration was significantly enhanced by 10-100 micrograms ml-1 in a dose-dependent manner. Moreover, saponin stimulated wound healing with enhanced angiogenesis in vivo.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Eriksson E., Rånby M., Gyzander E., Risberg B. Determination of plasminogen activator inhibitor in plasma using t-PA and a chromogenic single-point poly-D-lysine stimulated assay. Thromb Res. 1988 Apr 1;50(1):91–101. doi: 10.1016/0049-3848(88)90177-6. [DOI] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Houck K., Jakeman L., Leung D. W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992 Feb;13(1):18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki T., Ishikawa Y., Morisaki N., Shirai K., Saito Y., Yoshida S. Abnormal metabolism of polyunsaturated fatty acids and phospholipids in diabetic glomeruli. Lipids. 1987 Oct;22(10):704–710. doi: 10.1007/BF02533969. [DOI] [PubMed] [Google Scholar]
- Kawano M., Koshikawa T., Kanzaki T., Morisaki N., Saito Y., Yoshida S. Diabetes mellitus induces accelerated growth of aortic smooth muscle cells: association with overexpression of PDGF beta-receptors. Eur J Clin Invest. 1993 Feb;23(2):84–90. doi: 10.1111/j.1365-2362.1993.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa I., Yoshikawa M., Yoshihara M., Hayashi T., Taniyama T. [Chemical studies of crude drugs (1). Constituents of Ginseng radix rubra]. Yakugaku Zasshi. 1983 Jun;103(6):612–622. [PubMed] [Google Scholar]
- Koyama N., Harada K., Yamamoto A., Morisaki N., Saito Y., Yoshida S. Purification and characterization of an autocrine migration factor for vascular smooth muscle cells (SMC), SMC-derived migration factor. J Biol Chem. 1993 Jun 25;268(18):13301–13308. [PubMed] [Google Scholar]
- Koyama N., Koshikawa T., Morisaki N., Saito Y., Yoshida S. Secretion of a potent new migration factor for smooth muscle cells (SMC) by cultured SMC. Atherosclerosis. 1991 Feb;86(2-3):219–226. doi: 10.1016/0021-9150(91)90218-r. [DOI] [PubMed] [Google Scholar]
- Koyama N., Morisaki N., Saito Y., Yoshida S. Inhibitory effect of ginsenosides on migration of arterial smooth muscle cells. Am J Chin Med. 1992;20(2):167–173. doi: 10.1142/S0192415X92000175. [DOI] [PubMed] [Google Scholar]
- Koyama N., Morisaki N., Saito Y., Yoshida S. Regulatory effects of platelet-derived growth factor-AA homodimer on migration of vascular smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22806–22812. [PubMed] [Google Scholar]
- Koyama N., Watanabe S., Tezuka M., Morisaki N., Saito Y., Yoshida S. Migratory and proliferative effect of platelet-derived growth factor in rabbit retinal endothelial cells: evidence of an autocrine pathway of platelet-derived growth factor. J Cell Physiol. 1994 Jan;158(1):1–6. doi: 10.1002/jcp.1041580102. [DOI] [PubMed] [Google Scholar]
- Mawatari M., Okamura K., Matsuda T., Hamanaka R., Mizoguchi H., Higashio K., Kohno K., Kuwano M. Tumor necrosis factor and epidermal growth factor modulate migration of human microvascular endothelial cells and production of tissue-type plasminogen activator and its inhibitor. Exp Cell Res. 1991 Feb;192(2):574–580. doi: 10.1016/0014-4827(91)90078-9. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Morisaki N., Kanzaki T., Fujiyama Y., Osawa I., Shirai K., Matsuoka N., Saito Y., Yoshida S. Metabolism of n-3 polyunsaturated fatty acids and modification of phospholipids in cultured rabbit aortic smooth muscle cells. J Lipid Res. 1985 Aug;26(8):930–939. [PubMed] [Google Scholar]
- Morisaki N., Kanzaki T., Koshikawa T., Saito Y., Yoshida S. Secretion of a new growth factor, smooth muscle cell derived growth factor, distinct from platelet derived growth factor by cultured rabbit aortic smooth muscle cells. FEBS Lett. 1988 Mar 28;230(1-2):186–190. doi: 10.1016/0014-5793(88)80668-9. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Tezuka M., Koyama N., Morisaki N., Saito Y., Yoshida S., Araki N., Horiuchi S. Angiogenic effects of advanced glycation end products of the Maillard reaction on cultured human umbilical cord vein endothelial cells. Biochem Biophys Res Commun. 1993 Jun 15;193(2):674–680. doi: 10.1006/bbrc.1993.1677. [DOI] [PubMed] [Google Scholar]
- Tsuji T., Karasek M. A. A new method for obtaining scanning electron microscopic images of the reorganization process of human dermal microvascular endothelium in vitro. J Microsc. 1985 Jan;137(Pt 1):75–83. doi: 10.1111/j.1365-2818.1985.tb02563.x. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Binnema D., Scheffer M. A., Sprengers E. D., Kooistra T., Rijken D. C. Production of plasminogen activators and inhibitor by serially propagated endothelial cells from adult human blood vessels. Arteriosclerosis. 1987 Jul-Aug;7(4):389–400. doi: 10.1161/01.atv.7.4.389. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W. Regulation of the synthesis and secretion of plasminogen activators by endothelial cells. Haemostasis. 1988;18(4-6):307–327. doi: 10.1159/000215814. [DOI] [PubMed] [Google Scholar]