Abstract
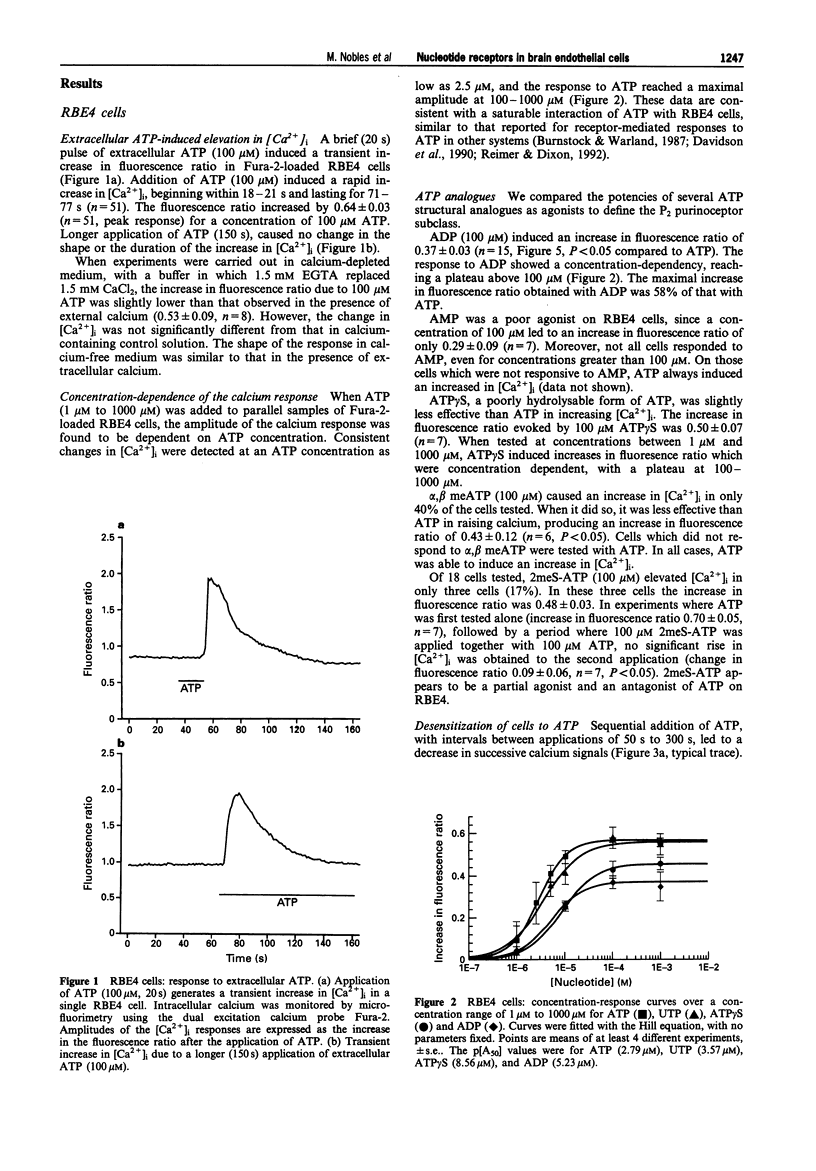
1. A dual-wavelength microfluorimetric method using Fura-2 as calcium indicator was applied to cells from an immortalized cell line of rat brain microvascular endothelial cells (RBE4), and to primary cultured rat brain endothelial cells. 2. In RBE4 cells, a brief (20 s) pulse of extracellular ATP (100 microM) induced a transient increase in the cytoplasmic calcium level ([Ca2+]i). Control responses to 100 microM ATP consisted of a ratio increase of 0.64 +/- 0.03 (mean +/- s.e., n = 51). Responses were seen at a concentration of 2.5 microM and were maximal at 100-1000 microM. When extracellular calcium was chelated with EGTA, the transient increase in [Ca2+]i was not affected. The results are consistent with Ca2+ mobilization from intracellular stores. 3. The purinoceptor involved belongs to the P2 subtype, since the agonist potency order among the adenine nucleotides was ATP > ADP > AMP. Moreover, the increase in [Ca2+]i evoked by ATP was partially inhibited by the P2 antagonist, suramin but was not affected by 8-phenyltheophylline, a P1-purinoceptor antagonist. The strong desensitization observed with repeated applications of ATP is also typical of a P2 receptor. 4. 2-Methylthio-ATP (2meS-ATP 100 microM), a P2Y agonist, elevated [Ca2+]i in only 17% of the cells tested; however, 2meS-ATP was found to antagonize the effect of ATP in all cells tested. The increase in [Ca2+]i evoked by ATP was inhibited by 500 s application of the P2Y purinoceptor antagonist, Reactive Blue 2 at 10 microM, while 60 s application of 100 microM was ineffective.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

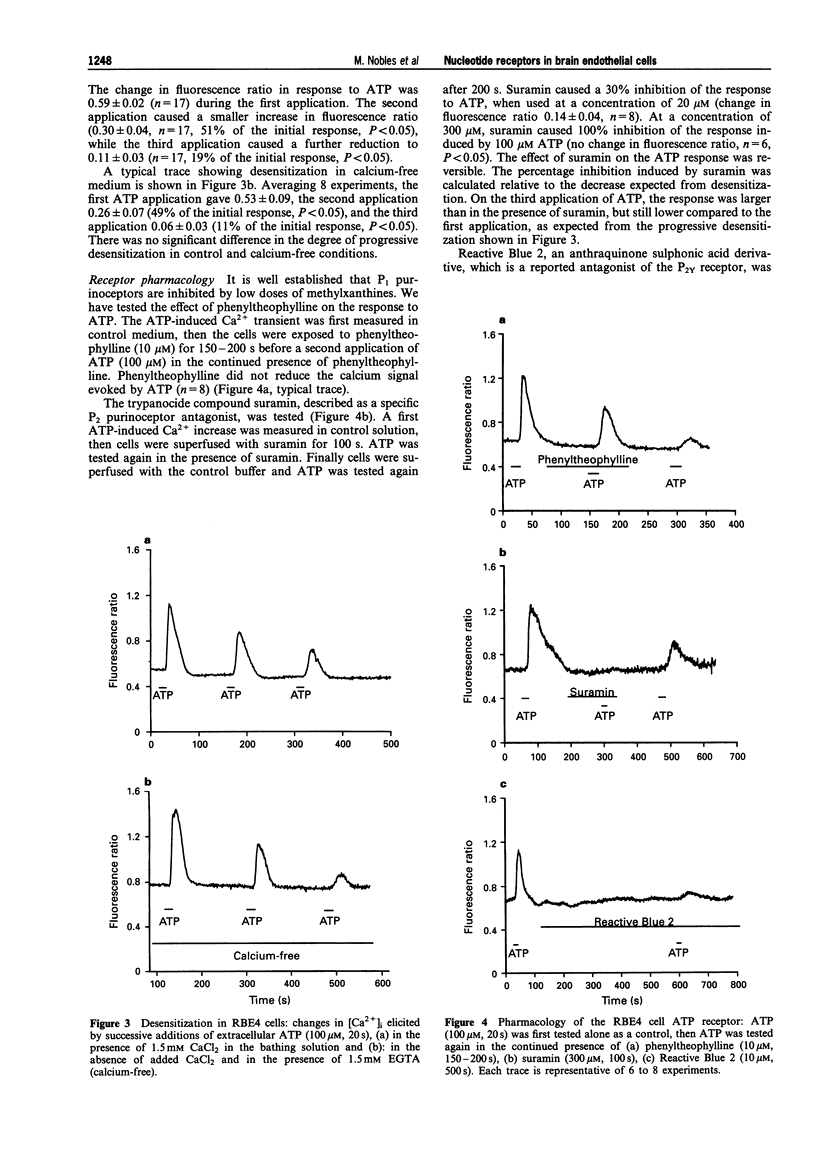


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott N. J., Hughes C. C., Revest P. A., Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992 Sep;103(Pt 1):23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Cusack N. J., Pearson J. D. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988 Dec;95(4):1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen D. S., Lazarus H. M., Shurin S. B., Stoll S. E., Dubyak G. R. Extracellular adenosine triphosphate activates calcium mobilization in human phagocytic leukocytes and neutrophil/monocyte progenitor cells. J Clin Invest. 1989 May;83(5):1651–1660. doi: 10.1172/JCI114064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E. Modulation of venular microvessel permeability by calcium influx into endothelial cells. FASEB J. 1992 Apr;6(7):2456–2466. doi: 10.1096/fasebj.6.7.1563597. [DOI] [PubMed] [Google Scholar]
- Davidson J. S., Wakefield I. K., Sohnius U., van der Merwe P. A., Millar R. P. A novel extracellular nucleotide receptor coupled to phosphoinositidase-C in pituitary cells. Endocrinology. 1990 Jan;126(1):80–87. doi: 10.1210/endo-126-1-80. [DOI] [PubMed] [Google Scholar]
- Doering A. E., Lederer W. J. The action of Na+ as a cofactor in the inhibition by cytoplasmic protons of the cardiac Na(+)-Ca2+ exchanger in the guinea-pig. J Physiol. 1994 Oct 1;480(Pt 1):9–20. doi: 10.1113/jphysiol.1994.sp020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieu-Trautmann O., Bourdoulous S., Roux F., Bourre J. M., Strosberg A. D., Couraud P. O. Immortalized rat brain microvessel endothelial cells: II--Pharmacological characterization. Adv Exp Med Biol. 1993;331:205–210. doi: 10.1007/978-1-4615-2920-0_32. [DOI] [PubMed] [Google Scholar]
- Durieu-Trautmann O., Fédérici C., Créminon C., Foignant-Chaverot N., Roux F., Claire M., Strosberg A. D., Couraud P. O. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol. 1993 Apr;155(1):104–111. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J. ATP--a fast neurotransmitter. FEBS Lett. 1993 Jun 28;325(1-2):86–89. doi: 10.1016/0014-5793(93)81419-z. [DOI] [PubMed] [Google Scholar]
- Frelin C., Breittmayer J. P., Vigne P. ADP induces inositol phosphate-independent intracellular Ca2+ mobilization in brain capillary endothelial cells. J Biol Chem. 1993 Apr 25;268(12):8787–8792. [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Hall D. A., Hourani S. M. Effects of analogues of adenine nucleotides on increases in intracellular calcium mediated by P2T-purinoceptors on human blood platelets. Br J Pharmacol. 1993 Mar;108(3):728–733. doi: 10.1111/j.1476-5381.1993.tb12869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P., Pagakis S. N., Curry F. E. Measurement of cytoplasmic calcium in single microvessels with increased permeability. Am J Physiol. 1990 May;258(5 Pt 2):H1366–H1374. doi: 10.1152/ajpheart.1990.258.5.H1366. [DOI] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993 Feb;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Kastritsis C. H., Salm A. K., McCarthy K. Stimulation of the P2Y purinergic receptor on type 1 astroglia results in inositol phosphate formation and calcium mobilization. J Neurochem. 1992 Apr;58(4):1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Kirkpatrick K. A., Burnstock G. Evidence that the inhibition of ATP release from sympathetic nerves by adenosine is a physiological mechanism. Gen Pharmacol. 1992 Nov;23(6):1045–1050. doi: 10.1016/0306-3623(92)90284-q. [DOI] [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoski N. S., Walz W. Ionic dependence of a P2-purinoceptor mediated depolarization of cultured astrocytes. J Neurosci Res. 1992 Aug;32(4):530–538. doi: 10.1002/jnr.490320408. [DOI] [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- O'Connor S. E. Recent developments in the classification and functional significance of receptors for ATP and UTP, evidence for nucleotide receptors. Life Sci. 1992;50(22):1657–1664. doi: 10.1016/0024-3205(92)90420-t. [DOI] [PubMed] [Google Scholar]
- Pearce B., Langley D. Purine- and pyrimidine-stimulated phosphoinositide breakdown and intracellular calcium mobilisation in astrocytes. Brain Res. 1994 Oct 17;660(2):329–332. doi: 10.1016/0006-8993(94)91307-2. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Burnstock G. Effects of purines and pyrimidines on the rat mesenteric arterial bed. Circ Res. 1991 Dec;69(6):1583–1590. doi: 10.1161/01.res.69.6.1583. [DOI] [PubMed] [Google Scholar]
- Reimer W. J., Dixon S. J. Extracellular nucleotides elevate [Ca2+]i in rat osteoblastic cells by interaction with two receptor subtypes. Am J Physiol. 1992 Nov;263(5 Pt 1):C1040–C1048. doi: 10.1152/ajpcell.1992.263.5.C1040. [DOI] [PubMed] [Google Scholar]
- Revest P. A., Abbott N. J., Gillespie J. I. Receptor-mediated changes in intracellular [Ca2+] in cultured rat brain capillary endothelial cells. Brain Res. 1991 May 17;549(1):159–161. doi: 10.1016/0006-8993(91)90614-2. [DOI] [PubMed] [Google Scholar]
- Roux F., Durieu-Trautmann O., Bourre J. M., Strosberg A. D., Couraud P. O. Immortalized rat brain microvessel endothelial cells: I--Expression of blood-brain barrier markers during angiogenesis. Adv Exp Med Biol. 1993;331:201–204. doi: 10.1007/978-1-4615-2920-0_31. [DOI] [PubMed] [Google Scholar]
- Roux F., Durieu-Trautmann O., Chaverot N., Claire M., Mailly P., Bourre J. M., Strosberg A. D., Couraud P. O. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1994 Apr;159(1):101–113. doi: 10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- Schlicker E., Urbanek E., Göthert M. ATP, alpha,beta-methylene ATP and suramin as tools for characterization of vascular P2x receptors in the pithed rat. J Auton Pharmacol. 1989 Oct;9(5):357–366. doi: 10.1111/j.1474-8673.1989.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Seifert R., Schultz G. Involvement of pyrimidinoceptors in the regulation of cell functions by uridine and by uracil nucleotides. Trends Pharmacol Sci. 1989 Sep;10(9):365–369. doi: 10.1016/0165-6147(89)90009-6. [DOI] [PubMed] [Google Scholar]
- Toda N., Ayajiki K., Okamura T. Cerebroarterial relaxations mediated by nitric oxide derived from endothelium and vasodilator nerve. J Vasc Res. 1993 Mar-Apr;30(2):61–67. doi: 10.1159/000158976. [DOI] [PubMed] [Google Scholar]
- Vigne P., Feolde E., Breittmayer J. P., Frelin C. Characterization of the effects of 2-methylthio-ATP and 2-chloro-ATP on brain capillary endothelial cells: similarities to ADP and differences from ATP. Br J Pharmacol. 1994 Jul;112(3):775–780. doi: 10.1111/j.1476-5381.1994.tb13146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund N. P., Gustafsson L. E. Indications for P2-purinoceptor subtypes in guinea pig smooth muscle. Eur J Pharmacol. 1988 Apr 13;148(3):361–370. doi: 10.1016/0014-2999(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Yu H. X., Turner J. T. Functional studies in the human submandibular duct cell line, HSG-PA, suggest a second salivary gland receptor subtype for nucleotides. J Pharmacol Exp Ther. 1991 Dec;259(3):1344–1350. [PubMed] [Google Scholar]


