Abstract
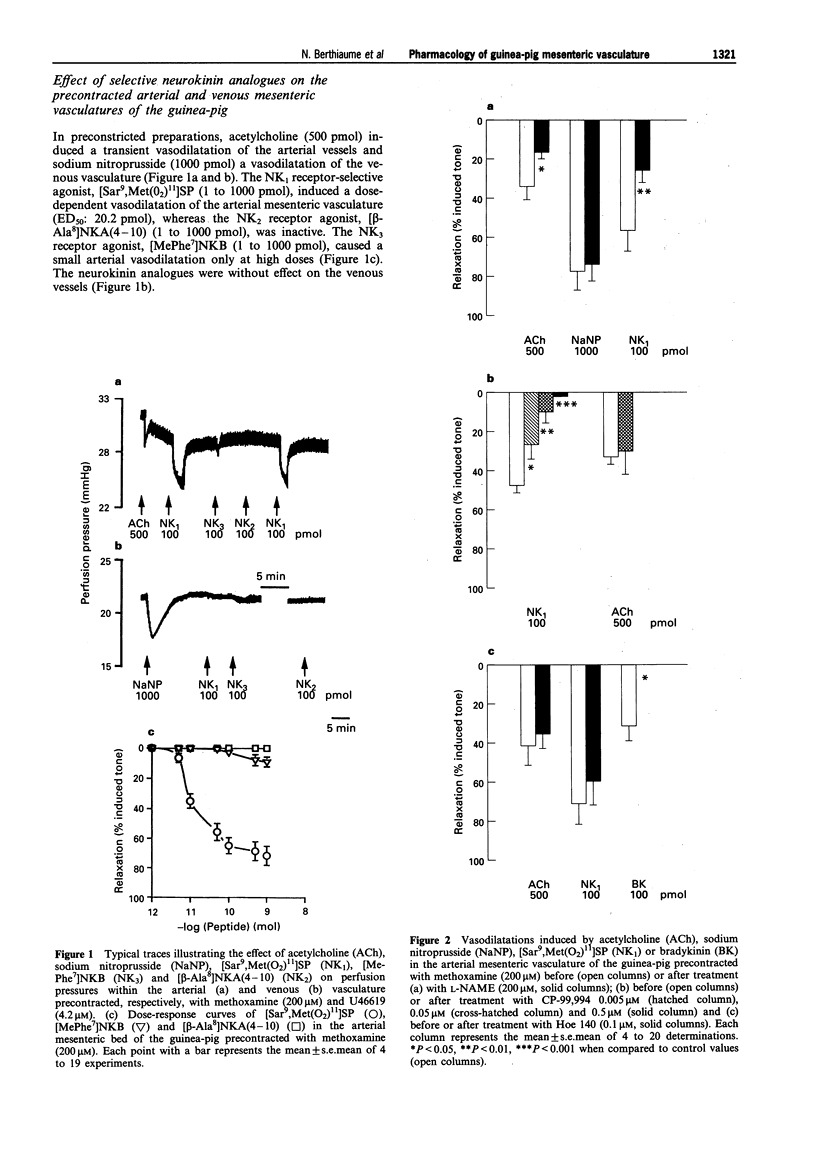
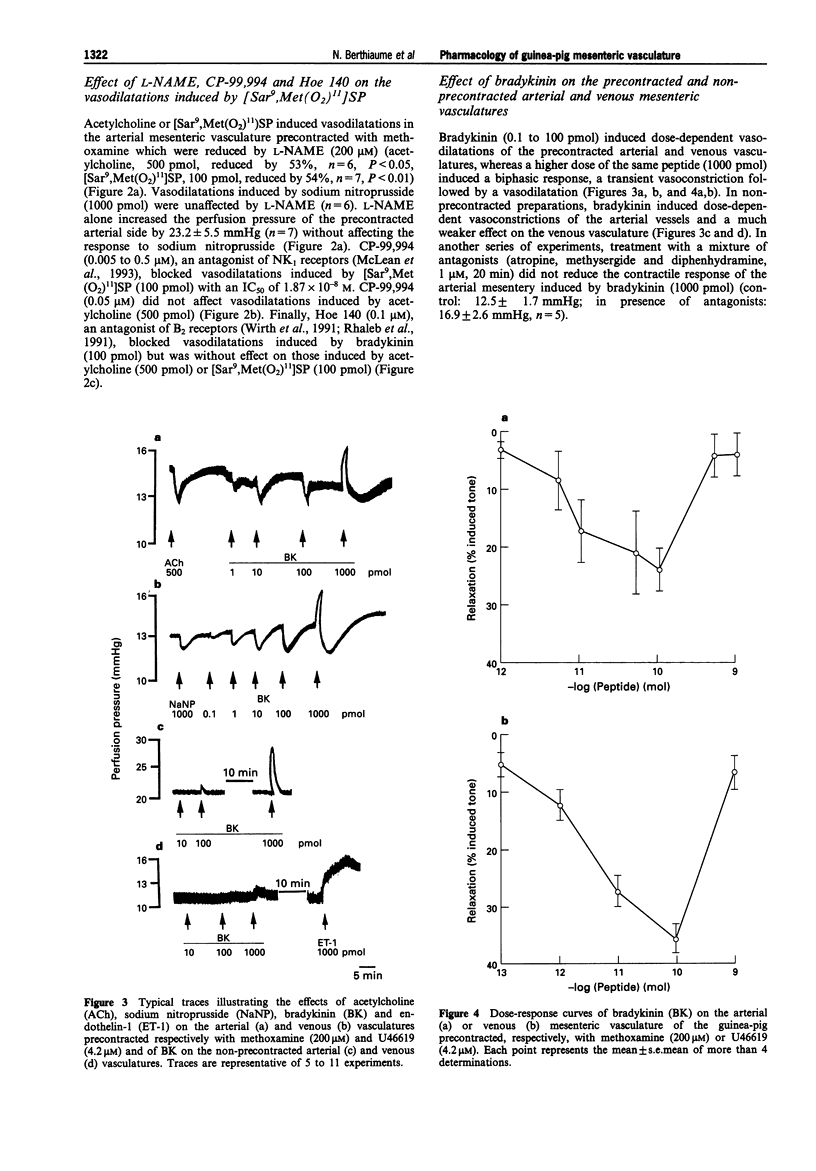
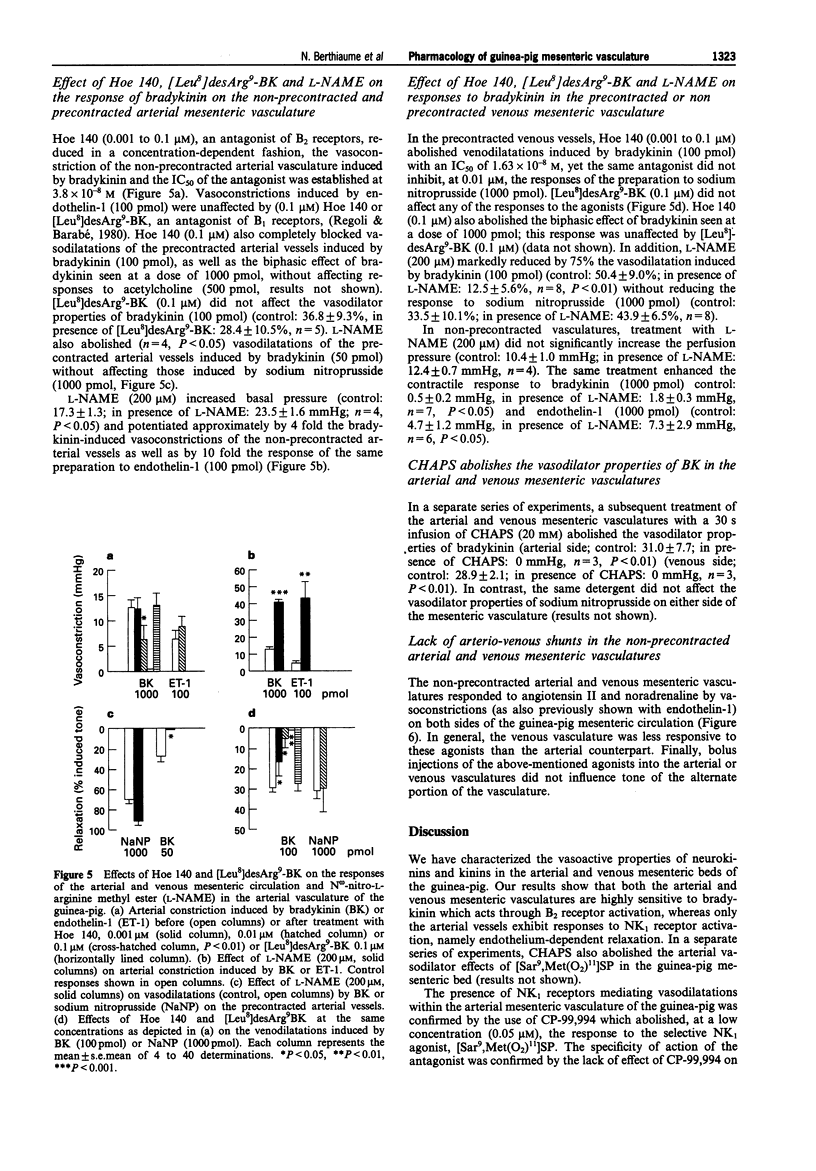
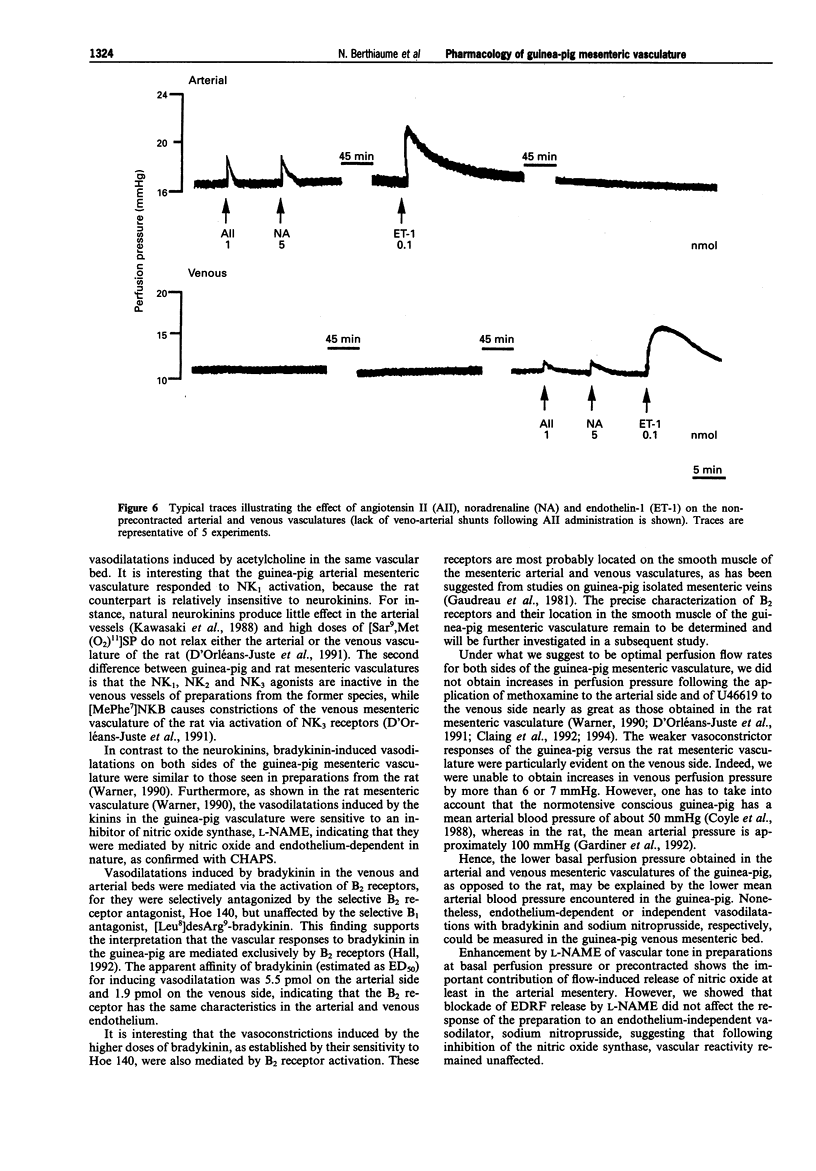
1. In the present work, we have studied the microvascular reactivity of the arterial and venous mesenteric beds of the guinea-pig to bradykinin, neurokinins and other agents. 2. The vasoactive properties of three selective agonists for neurokinin receptors, namely [Sar9, Met (O2)11]SP (NK1), [beta-Ala8]NKA(4-10) (NK2) and [MePhe7]NKB (NK3), were evaluated on precontracted arterial and venous mesenteric vasculatures of the guinea-pig. The NK1-selective agonist, [Sar9,Met(O2)11]SP (1 to 1000 pmol), induced an endothelium-dependent and N omega-nitro-L-arginine methyl ester (L-NAME)-sensitive relaxation of the arterial vasculature precontracted with methoxamine, whereas the NK2 and NK3-selective agonists were virtually inactive at high doses (1000 pmol). 3. The three selective neurokinin receptor agonists were inactive in the non-precontracted arterial and venous mesenteric vasculatures as well as in the precontracted venous mesenteric vasculature. 4. Bradykinin (0.1 to 100 pmol) induced a marked dose- and endothelium-dependent vasodilatation of the precontracted arterial and venous vasculatures. ED50 values were 5.5 pmol on the arterial side and 1.9 pmol on the venous side. In contrast, desArg9-bradykinin was inactive at doses up to 1000 pmol. Furthermore, on the arterial and venous sides, a higher dose of bradykinin (1000 pmol), induced a biphasic effect, a transient constriction followed by a marked and sustained vasodilatation. The vasodilator effects of bradykinin were abolished by Hoe 140 (0.1 microM) and CHAPS, markedly reduced by L-NAME and were unaffected by [Leu8]desArg9-bradykinin (0.1 microM) on both sides of the mesenteric vasculature. Hoe 140 also abolished the arterial vasoconstrictions induced by high doses of bradykinin.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claing A., Bkaily G., Berthiaume N., Sirois P., Rola-Pleszczynski M., D'Orléans-Juste P. Role of R-type calcium channels in the response of the perfused arterial and venous mesenteric vasculature of the rat to platelet-activating factor. Br J Pharmacol. 1994 Aug;112(4):1202–1208. doi: 10.1111/j.1476-5381.1994.tb13211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing A., Télémaque S., Cadieux A., Fournier A., Regoli D., D'Orléans-Juste P. Nonadrenergic and noncholinergic arterial dilatation and venoconstriction are mediated by calcitonin gene-related peptide1 and neurokinin-1 receptors, respectively, in the mesenteric vasculature of the rat after perivascular nerve stimulation. J Pharmacol Exp Ther. 1992 Dec;263(3):1226–1232. [PubMed] [Google Scholar]
- Coyle A. J., Urwin S. C., Page C. P., Touvay C., Villain B., Braquet P. The effect of the selective PAF antagonist BN 52021 on PAF- and antigen-induced bronchial hyper-reactivity and eosinophil accumulation. Eur J Pharmacol. 1988 Mar 22;148(1):51–58. doi: 10.1016/0014-2999(88)90453-0. [DOI] [PubMed] [Google Scholar]
- D'Orléans-Juste P., Claing A., Télémaque S., Warner T. D., Regoli D. Neurokinins produce selective venoconstriction via NK-3 receptors in the rat mesenteric vascular bed. Eur J Pharmacol. 1991 Nov 12;204(3):329–334. doi: 10.1016/0014-2999(91)90860-s. [DOI] [PubMed] [Google Scholar]
- D'Orléans-Juste P., Claing A., Warner T. D., Yano M., Télémaque S. Characterization of receptors for endothelins in the perfused arterial and venous mesenteric vasculatures of the rat. Br J Pharmacol. 1993 Oct;110(2):687–692. doi: 10.1111/j.1476-5381.1993.tb13866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G., Regoli D. Synthesis of bradykinin analogs. Methods Enzymol. 1988;163:263–272. doi: 10.1016/0076-6879(88)63025-4. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Kemp P. A., Bennett T., Bose C., Foulkes R., Hughes B. Involvement of beta 2-adrenoceptors in the regional haemodynamic responses to bradykinin in conscious rats. Br J Pharmacol. 1992 Apr;105(4):839–848. doi: 10.1111/j.1476-5381.1992.tb09066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreau P., Barabé J., St-Pierre S., Regoli D. Structure-activity study of kinins in vascular smooth muscles. Can J Physiol Pharmacol. 1981 Apr;59(4):380–389. doi: 10.1139/y81-060. [DOI] [PubMed] [Google Scholar]
- Hall J. M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992 Nov;56(2):131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Takasaki K., Saito A., Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988 Sep 8;335(6186):164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- MCGREGOR D. D. THE EFFECT OF SYMPATHETIC NERVE STIMULATION OF VASOCONSTRICTOR RESPONSES IN PERFUSED MESENTERIC BLOOD VESSELS OF THE RAT. J Physiol. 1965 Mar;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S., Snider R. M., Desai M. C., Rosen T., Bryce D. K., Longo K. P., Schmidt A. W., Heym J. CP-99,994, a nonpeptide antagonist of the tachykinin NK1 receptor. Regul Pept. 1993 Jul 2;46(1-2):329–331. doi: 10.1016/0167-0115(93)90075-j. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Rhaleb N. E., Télémaque S., Rouissi N., Dion S., Jukic D., Drapeau G., Regoli D. Structure-activity studies of bradykinin and related peptides. B2-receptor antagonists. Hypertension. 1991 Jan;17(1):107–115. doi: 10.1161/01.hyp.17.1.107. [DOI] [PubMed] [Google Scholar]
- Suh S. H., Chen G., Xue L., Zhang G., Yamamoto Y., Suzuki H. Inhibitory actions of MCI-154 on guinea-pig femoral artery and vein preparations. Eur J Pharmacol. 1992 Sep 4;219(3):377–383. doi: 10.1016/0014-2999(92)90478-m. [DOI] [PubMed] [Google Scholar]
- Warner T. D., D'Orleans-Juste P., Vane J. R. Endothelin-1 and U46619 potentiate selectively the venous responses to nerve stimulation within the perfused superior mesenteric vascular bed of the rat. Biochem Biophys Res Commun. 1990 Oct 30;172(2):745–750. doi: 10.1016/0006-291x(90)90737-8. [DOI] [PubMed] [Google Scholar]
- Warner T. D., Mitchell J. A., de Nucci G., Vane J. R. Endothelin-1 and endothelin-3 release EDRF from isolated perfused arterial vessels of the rat and rabbit. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S85–S102. doi: 10.1097/00005344-198900135-00021. [DOI] [PubMed] [Google Scholar]
- Warner T. D. Simultaneous perfusion of rat isolated superior mesenteric arterial and venous beds: comparison of their vasoconstrictor and vasodilator responses to agonists. Br J Pharmacol. 1990 Feb;99(2):427–433. doi: 10.1111/j.1476-5381.1990.tb14720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth K., Hock F. J., Albus U., Linz W., Alpermann H. G., Anagnostopoulos H., Henk S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br J Pharmacol. 1991 Mar;102(3):774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


