Abstract
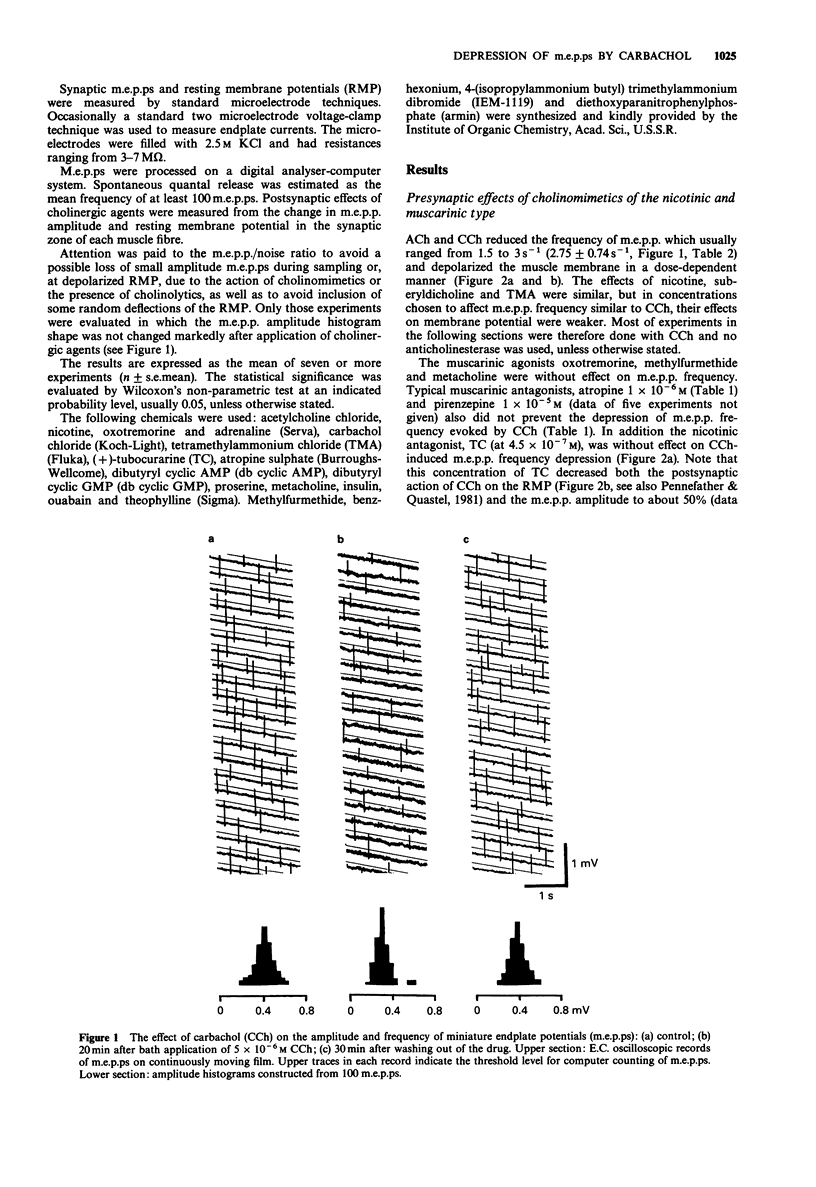
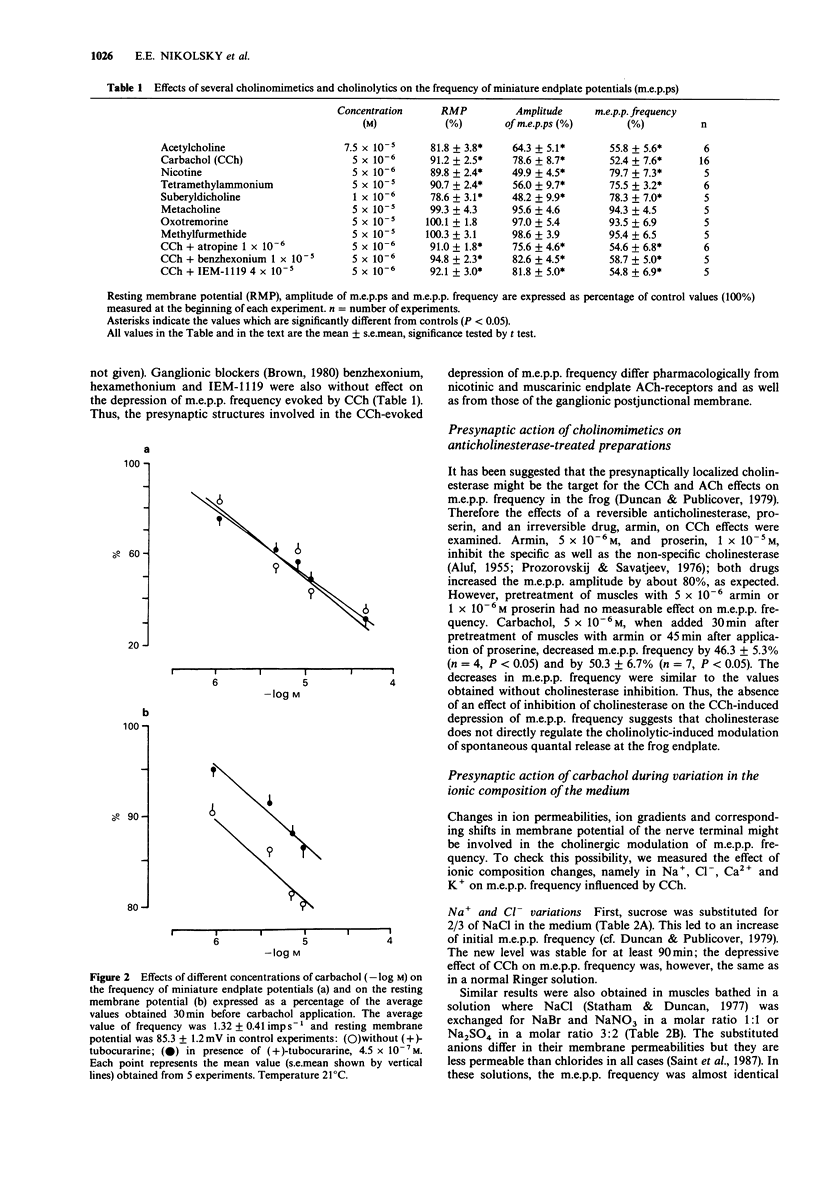
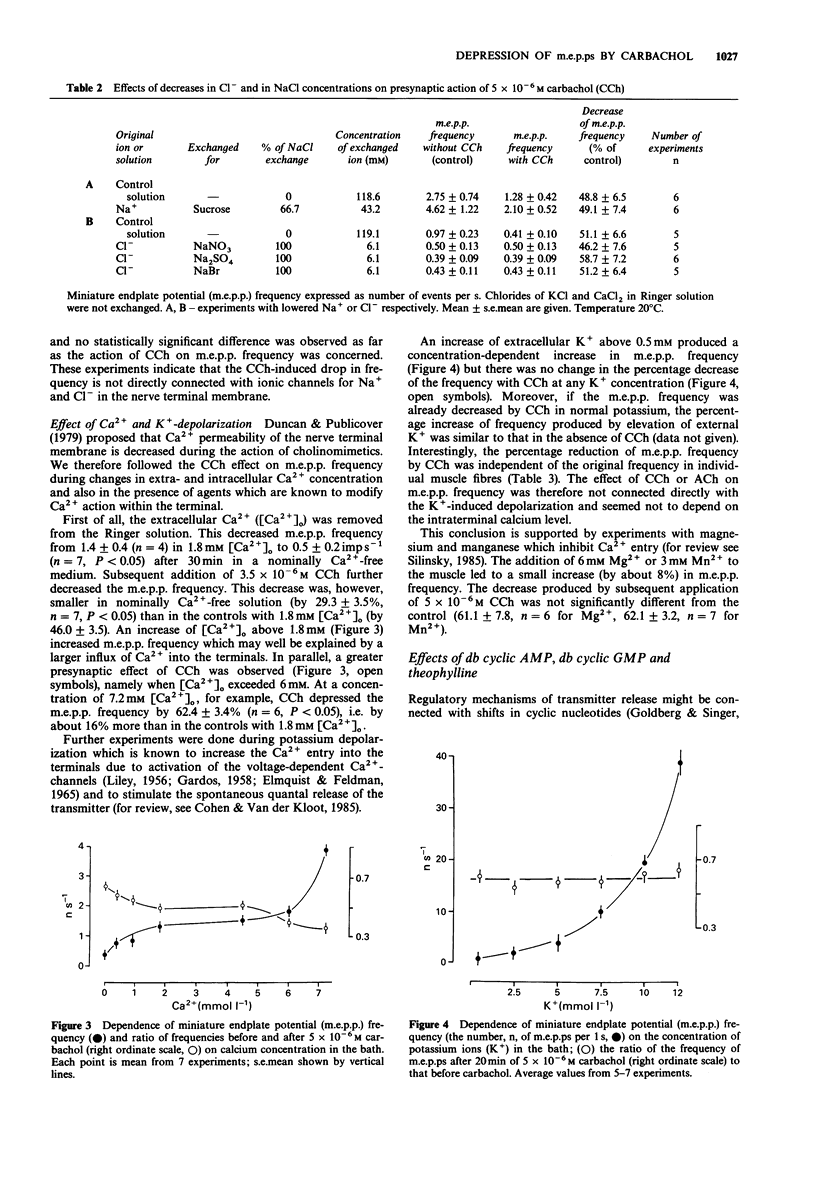
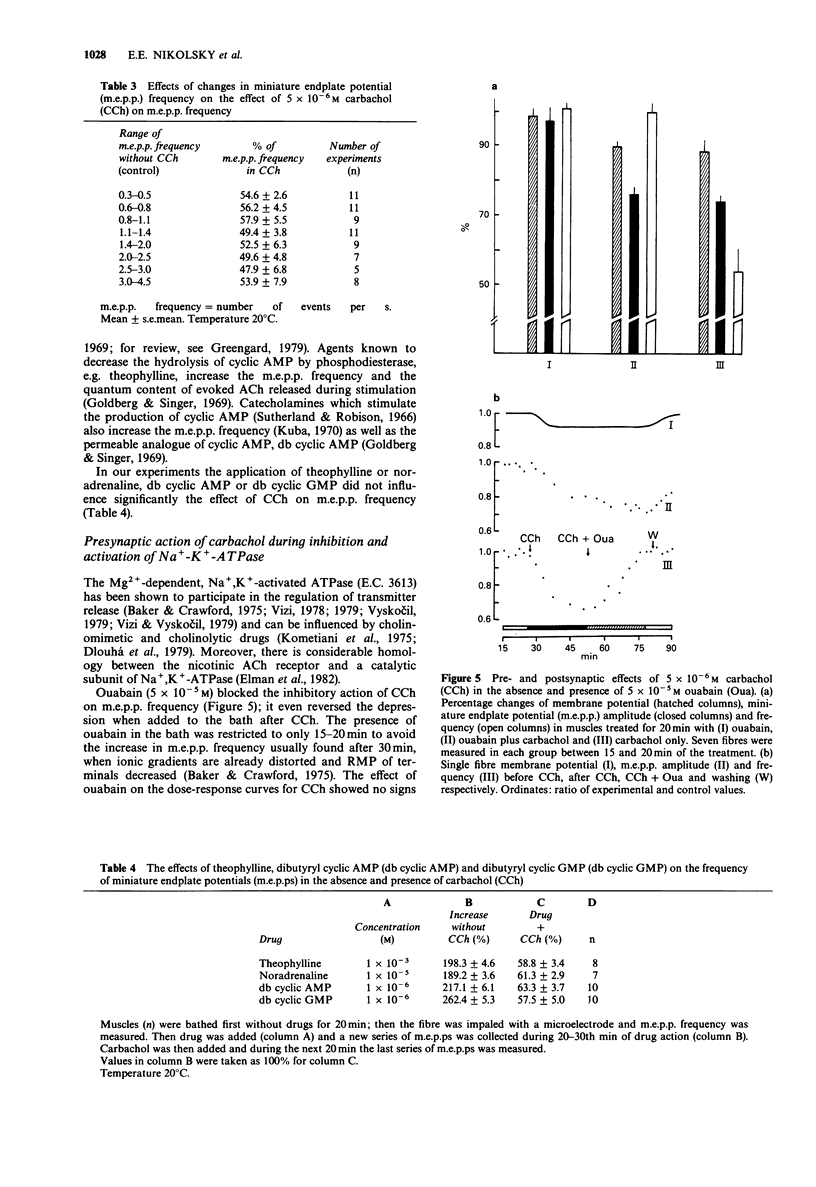
1. Acetylcholine (ACh), 7.5 x 10(-5) M, and carbachol, 5 x 10(-6) M (CCh) depressed the frequency of miniature endplate potentials (m.e.p.ps) in the frog (Rana temporaria) sartorius neuromuscular junction with active acetylcholinesterase to about 50-55% of the controls. 2. A similar depression was produced by the nicotinic agonists, nicotine, suberyldicholine and tetramethylammonium. 3. The muscarinic agonists, oxotremorine, methylfurmethide and methacholine were without effect on m.e.p.p. frequency. The muscarinic antagonist, atropine and the nicotinic antagonist, (+)-tubocurarine, had no effect on the depression of m.e.p.p. frequency evoked by CCh. 4. The ganglionic blockers, benzhexonium and IEM-1119, were also without effect on the CCh-evoked depression of m.e.p.p. frequency. 5. Pretreatment of muscles with anticholinesterases did not prevent the CCh-induced drop in m.e.p.p. frequency. 6. The effect of CCh was proportionally the same as in the controls in preparations where the m.e.p.p. frequency was changed by elevation of K+ and in the presence of theophylline, noradrenaline, dibutyryl adenosine 3':5'-cyclic monophosphate (db cyclic AMP) and db cyclic GMP. 7. An inhibitor of Na+,K(+)-ATPase, ouabain, 5 x 10(-5) mol l-1, prevented or reversed the depression of m.e.p.p. frequency by CCh. However, the depression was present in a nominally K(+)-free medium. Insulin and adrenaline, which are considered to be Na+,K(+)-ATPase activators, were without effect on depression of m.e.p.p. frequency. 8. The depression of m.e.p.p. frequency by 5 x 10(-6) M CCh was the same at temperatures between 5 and 30 degrees C with a Q10 near to 1.0.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALUF M. A. K farmokologii armina. Farmakol Toksikol. 1955 Mar-Apr;18(2):21–27. [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukharaeva E. A., Nikol'skii E. E., Giniatullin R. A. Vliianie kholinergicheskikh soedinenii na spontannoe kvantovoe osvobozhdenie mediatora v nervno-myshechnom sinapse liagushki. Neirofiziologiia. 1986;18(5):586–593. [PubMed] [Google Scholar]
- CIANI S., EDWARDS C. THE EFFECT OF ACETYLCHOLINE ON NEUROMUSCULAR TRANSMISSION IN THE FROG. J Pharmacol Exp Ther. 1963 Oct;142:21–23. [PubMed] [Google Scholar]
- Clausen T. Regulation of active Na+-K+ transport in skeletal muscle. Physiol Rev. 1986 Jul;66(3):542–580. doi: 10.1152/physrev.1986.66.3.542. [DOI] [PubMed] [Google Scholar]
- Cohen I., Van der Kloot W. Calcium and transmitter release. Int Rev Neurobiol. 1985;27:299–336. doi: 10.1016/s0074-7742(08)60560-7. [DOI] [PubMed] [Google Scholar]
- Dlouhá H., Teisinger J., Vyskocil F. Activation of membrane Na+/K+-ATPase of mouse skeletal muscle by acetylcholine and its inhibition by alpha-bungarotoxin, curare and atropine. Pflugers Arch. 1979 May 15;380(1):101–104. doi: 10.1007/BF00582620. [DOI] [PubMed] [Google Scholar]
- Duncan C. J., Publicover S. J. Inhibitory effects of cholinergic agents on the release of transmitter at the frog neuromuscular junction. J Physiol. 1979 Sep;294:91–103. doi: 10.1113/jphysiol.1979.sp012917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfman L., Heilbronn E., Jørgensen P. L. Fractionation of protein components of plasma membranes from the electric organ of Torpedo marmorata. Biochim Biophys Acta. 1982 Dec 22;693(2):273–279. doi: 10.1016/0005-2736(82)90432-1. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., MOORE L. E. THE EFFECT OF TEMPERATURE ON THE SODIUM AND POTASSIUM PERMEABILITY CHANGES IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:431–437. doi: 10.1113/jphysiol.1963.sp007269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Singer J. J. Evidence for a role of cyclic AMP in neuromuscular transmission. Proc Natl Acad Sci U S A. 1969 Sep;64(1):134–141. doi: 10.1073/pnas.64.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimann C., Torri-Tarelli F., Fesce R., Ceccarelli B. Measurement of quantal secretion induced by ouabain and its correlation with depletion of synaptic vesicles. J Cell Biol. 1985 Nov;101(5 Pt 1):1953–1965. doi: 10.1083/jcb.101.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Sterz R., Peper K. Prejunctional effects of anticholinesterase drugs at the endplate: mediated by presynaptic acetylcholine receptors or by postsynaptic potassium efflux? Pflugers Arch. 1981 Sep;391(3):213–218. doi: 10.1007/BF00596173. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- Kijima H., Tanabe N. Calcium-independent increase of transmitter release at frog end-plate by trinitrobenzene sulphonic acid. J Physiol. 1988 Sep;403:135–149. doi: 10.1113/jphysiol.1988.sp017243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbinger H., Kruel R. Choline inhibits acetylcholine release via presynaptic muscarine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1981 Apr;316(2):131–134. doi: 10.1007/BF00505306. [DOI] [PubMed] [Google Scholar]
- Kuba K. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol. 1970 Dec;211(3):551–570. doi: 10.1113/jphysiol.1970.sp009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Nikol'skii E. E. Pre- i postsinapticheskoe deistvie kholinomimetika (subekholina) v usloviiakh fiksatsii potentsiala na postsinapticheskoi membrane. Dokl Akad Nauk SSSR. 1979;249(6):1488–1491. [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Stimulation-induced factors which affect augmentation and potentiation of trasmitter release at the neuromuscular junction. J Physiol. 1976 Sep;260(3):687–717. doi: 10.1113/jphysiol.1976.sp011539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunaka Y. Effects of external K concentration on the electrogenicity of the insulin-stimulated Na,K-pump in frog skeletal muscle. J Membr Biol. 1986;91(2):165–172. doi: 10.1007/BF01925793. [DOI] [PubMed] [Google Scholar]
- McLarnon J. G., Saint D. A., Quastel D. M. The actions of dimethyl sulfoxide on neuromuscular transmission. Mol Pharmacol. 1986 Dec;30(6):631–638. [PubMed] [Google Scholar]
- Michaelson D. M., Avissar S., Kloog Y., Sokolovsky M. Mechanism of acetylcholine release: possible involvement of presynaptic muscarinic receptors in regulation of acetylcholine release and protein phosphorylation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6336–6340. doi: 10.1073/pnas.76.12.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. D., Volle R. L. Enhancement by carbachol of transmitter release from motor nerve terminals. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1489–1492. doi: 10.1073/pnas.71.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikol'skii E. E., Giniatullin R. A. Prekrashchenie presinapticheskogo deistiviia karbakholina tubokurarinom. Biull Eksp Biol Med. 1979 Feb;87(2):171–174. [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Relation between subsynaptic receptor blockade and response to quantal transmitter at the mouse neuromuscular junction. J Gen Physiol. 1981 Sep;78(3):313–344. doi: 10.1085/jgp.78.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Robbins N. Cation movements in normal and short-term denervated rat fast twitch muscle. J Physiol. 1977 Oct;271(3):605–624. doi: 10.1113/jphysiol.1977.sp012017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint D. A., McLarnon J. G., Quastel D. M. Anion permeability of motor nerve terminals. Pflugers Arch. 1987 Jul;409(3):258–264. doi: 10.1007/BF00583474. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Statham H. E., Duncan C. J. The effect of sodium ions of MEPP frequency at the frog neuromuscular junction. Life Sci. 1977 Jun 1;20(11):1839–1845. doi: 10.1016/0024-3205(77)90219-3. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Thesleff S., Molgó J., Lundh H. Botulinum toxin and 4-aminoquinoline induce a similar abnormal type of spontaneous quantal transmitter release at the rat neuromuscular junction. Brain Res. 1983 Mar 28;264(1):89–97. doi: 10.1016/0006-8993(83)91123-x. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Na+-K+-activated adenosinetriphosphatase as a trigger in transmitter release. Neuroscience. 1978;3(4-5):367–384. doi: 10.1016/0306-4522(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(3-4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Vyskocil F. Changes in total and quantal release of acetylcholine in the mouse diaphragm during activation and inhibition of membrane ATPase. J Physiol. 1979 Jan;286:1–14. doi: 10.1113/jphysiol.1979.sp012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Non-quantal release of transmitter at mouse neuromuscular junction and its dependence on the activity of Na+-K+ ATP-ase. Pflugers Arch. 1977 Sep 16;370(3):295–297. doi: 10.1007/BF00585542. [DOI] [PubMed] [Google Scholar]
- Vyskocil F. The regulatory role of membrane Na+-K+-ATPase in non-quantal release of transmitter at the neuromuscular junction. Prog Brain Res. 1979;49:183–189. doi: 10.1016/S0079-6123(08)64632-4. [DOI] [PubMed] [Google Scholar]
- Zemková H., Vyskocil F., Edwards C. The effects of nerve terminal activity on non-quantal release of acetylcholine at the mouse neuromuscular junction. J Physiol. 1990 Apr;423:631–640. doi: 10.1113/jphysiol.1990.sp018044. [DOI] [PMC free article] [PubMed] [Google Scholar]


