Abstract
1. Isolated melanophores were differentiated from aggregates of neural crest obtained from neurula stage Xenopus laevis embryos after 2 days in culture. 2. Condensation of pigment granules in these cells by melatonin (5-methoxy N-acetyltryptamine, aMT) and various novel analogues was monitored with an image analysis system to quantitate the area occupied by pigment in individual cells. 3. Melanophores exposed to vehicle (a maximum of 0.1% MeOH) showed little (less than 5%) change in pigment area. aMT produced a dramatic condensation of pigment granules (EC50 = the concentration producing a half maximal condensation, 9 pM). The response was rapid, reached a maximum (approximately 80% decrease in pigmented area) by 10 min, and was reversible after removal of aMT from the culture medium. 4. Aggregation to aMT was blocked by treating melanophores with pertussis toxin (1 microgram ml-1, 7 h) indicating a role for a guanosine 5' triphosphate (GTP)-binding protein in transducing the aMT receptor signal. 5. Structure-activity studies indicated that analogues of aMT lacking a side-chain N-acyl substituent (5-methoxytryptamine, MT) or a group at the 5-position of the indole ring (N-acetyltryptamine, aT) were unable to induce pigment aggregation (EC50 greater than 10 microM). 6. Lengthening the side-chain N-acyl group (N-propionyl, N-butanoyl) was tolerated to some degree but eventually (N-valeroyl and larger) activity diminished. Of the 5-position analogues tested 5-methoxy (aMT) was by far the most potent. 7. Halogen substitution in the 6-position of the indole ring led to some loss of activity as did a 6-OH substitution. The 6-OCH3 compound was inactive.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

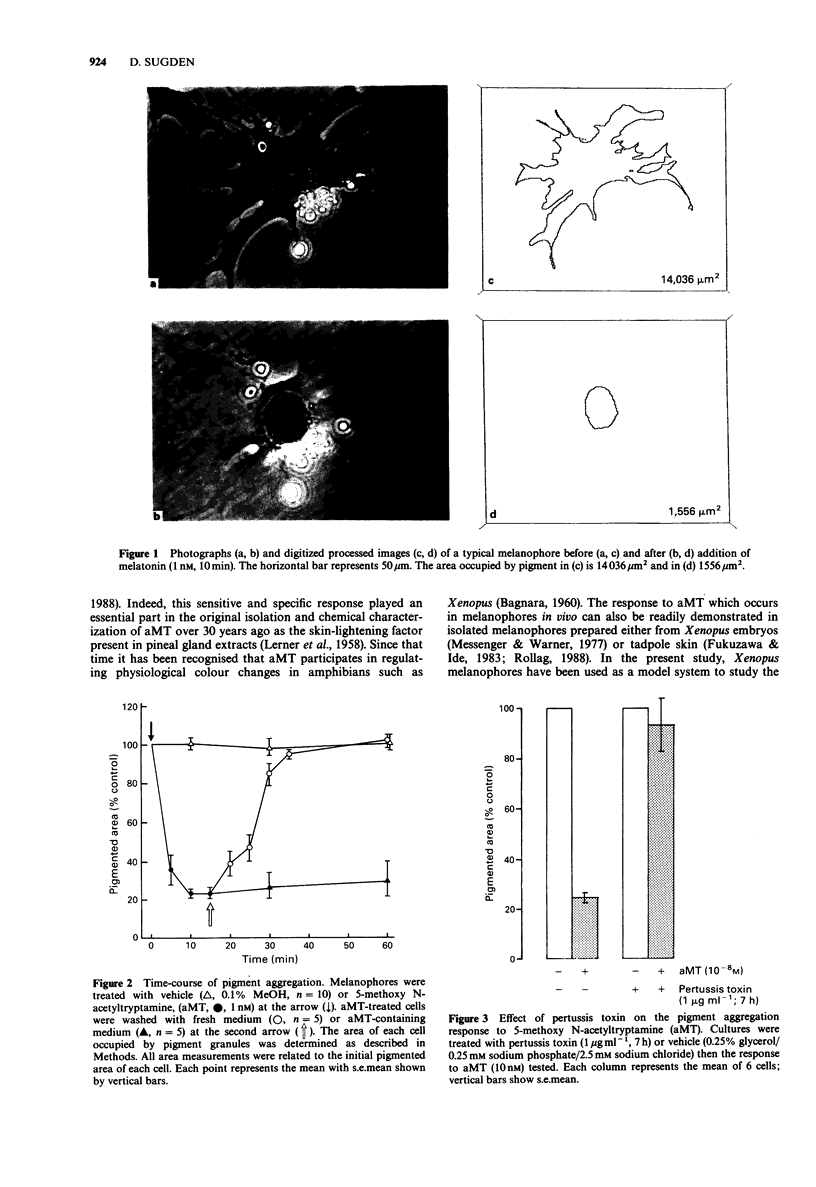


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Robison G. A., Liddle G. W., Butcher R. W., Nicholson W. E., Baird C. E. Role of cyclic AMP in mediating the effects of MSH, norepinephrine, and melatonin on frog skin color. Endocrinology. 1969 Oct;85(4):674–682. doi: 10.1210/endo-85-4-674. [DOI] [PubMed] [Google Scholar]
- Armstrong S. M. Melatonin and circadian control in mammals. Experientia. 1989 Oct 15;45(10):932–938. doi: 10.1007/BF01953050. [DOI] [PubMed] [Google Scholar]
- BAGNARA J. T. Pineal regulation of the body lightening reaction in amphibian larvae. Science. 1960 Nov 18;132(3438):1481–1483. doi: 10.1126/science.132.3438.1481-a. [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L. Pharmacology and function of melatonin receptors. FASEB J. 1988 Sep;2(12):2765–2773. doi: 10.1096/fasebj.2.12.2842214. [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L., Shankar G., Mickel M. 2-[125I]iodomelatonin labels sites with identical pharmacological characteristics in chicken brain and chicken retina. Eur J Pharmacol. 1989 Mar 21;162(2):289–299. doi: 10.1016/0014-2999(89)90292-6. [DOI] [PubMed] [Google Scholar]
- Duncan M. J., Takahashi J. S., Dubocovich M. L. 2-[125I]iodomelatonin binding sites in hamster brain membranes: pharmacological characteristics and regional distribution. Endocrinology. 1988 May;122(5):1825–1833. doi: 10.1210/endo-122-5-1825. [DOI] [PubMed] [Google Scholar]
- Duncan M. J., Takahashi J. S., Dubocovich M. L. Characteristics and autoradiographic localization of 2-[125I]iodomelatonin binding sites in Djungarian hamster brain. Endocrinology. 1989 Aug;125(2):1011–1018. doi: 10.1210/endo-125-2-1011. [DOI] [PubMed] [Google Scholar]
- Fukuzawa T., Ide H. Proliferation in vitro of melanophores from Xenopus laevis. J Exp Zool. 1983 May;226(2):239–244. doi: 10.1002/jez.1402260209. [DOI] [PubMed] [Google Scholar]
- Heward C. B., Hadley M. E. Structure-activity relationships of melatonin and related indoleamines. Life Sci. 1975 Oct 10;17(7):1167–1177. doi: 10.1016/0024-3205(75)90340-9. [DOI] [PubMed] [Google Scholar]
- Ho B. T., McIsaac W. M., Tansey L. W., Kralik P. M. Hydroxyindole-O-methyltransferase. II. Inhibitory activities of some N-acyltryptamines. J Pharm Sci. 1968 Nov;57(11):1998–2000. doi: 10.1002/jps.2600571139. [DOI] [PubMed] [Google Scholar]
- Laitinen J. T., Flügge G., Saavedra J. M. Characterization of melatonin receptors in the rat area postrema: modulation of affinity with cations and guanine nucleotides. Neuroendocrinology. 1990 Jun;51(6):619–624. doi: 10.1159/000125401. [DOI] [PubMed] [Google Scholar]
- Messenger E. A., Warner A. E. The action of melatonin on single amphibian pigment cells in tissue culture. Br J Pharmacol. 1977 Dec;61(4):607–614. doi: 10.1111/j.1476-5381.1977.tb07554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. J., Williams L. M. Central melatonin receptors: implications for a mode of action. Experientia. 1989 Oct 15;45(10):955–965. doi: 10.1007/BF01953053. [DOI] [PubMed] [Google Scholar]
- Quay W. B. Specificity and structure-activity relationships in the Xenopus larval melanophore assay for melatonin. Gen Comp Endocrinol. 1968 Aug;11(1):253–254. doi: 10.1016/0016-6480(68)90127-5. [DOI] [PubMed] [Google Scholar]
- Seldenrijk R., Hup D. R., de Graan P. N., van de Veerdonk F. C. Morphological and physiological aspects of melanophores in primary culture from tadpoles of Xenopus laevis. Cell Tissue Res. 1979 May 25;198(3):397–409. doi: 10.1007/BF00234185. [DOI] [PubMed] [Google Scholar]
- Sugden D., Chong N. W. Pharmacological identity of 2-[125I]iodomelatonin binding sites in chicken brain and sheep pars tuberalis. Brain Res. 1991 Jan 18;539(1):151–154. doi: 10.1016/0006-8993(91)90698-u. [DOI] [PubMed] [Google Scholar]
- Tamarkin L., Baird C. J., Almeida O. F. Melatonin: a coordinating signal for mammalian reproduction? Science. 1985 Feb 15;227(4688):714–720. doi: 10.1126/science.3881822. [DOI] [PubMed] [Google Scholar]
- Vakkuri O., Lämsä E., Rahkamaa E., Ruotsalainen H., Leppäluoto J. Iodinated melatonin: preparation and characterization of the molecular structure by mass and 1H NMR spectroscopy. Anal Biochem. 1984 Nov 1;142(2):284–289. doi: 10.1016/0003-2697(84)90466-4. [DOI] [PubMed] [Google Scholar]
- Vanecek J., Vollrath L. Melatonin inhibits cyclic AMP and cyclic GMP accumulation in the rat pituitary. Brain Res. 1989 Dec 25;505(1):157–159. doi: 10.1016/0006-8993(89)90129-7. [DOI] [PubMed] [Google Scholar]
- WRIGHT M. R., LERNER A. B. On the movement of pigment granules in frog melanocytes. Endocrinology. 1960 Apr;66:599–609. doi: 10.1210/endo-66-4-599. [DOI] [PubMed] [Google Scholar]
- White B. H., Sekura R. D., Rollag M. D. Pertussis toxin blocks melatonin-induced pigment aggregation in Xenopus dermal melanophores. J Comp Physiol B. 1987;157(2):153–159. doi: 10.1007/BF00692359. [DOI] [PubMed] [Google Scholar]



