Abstract
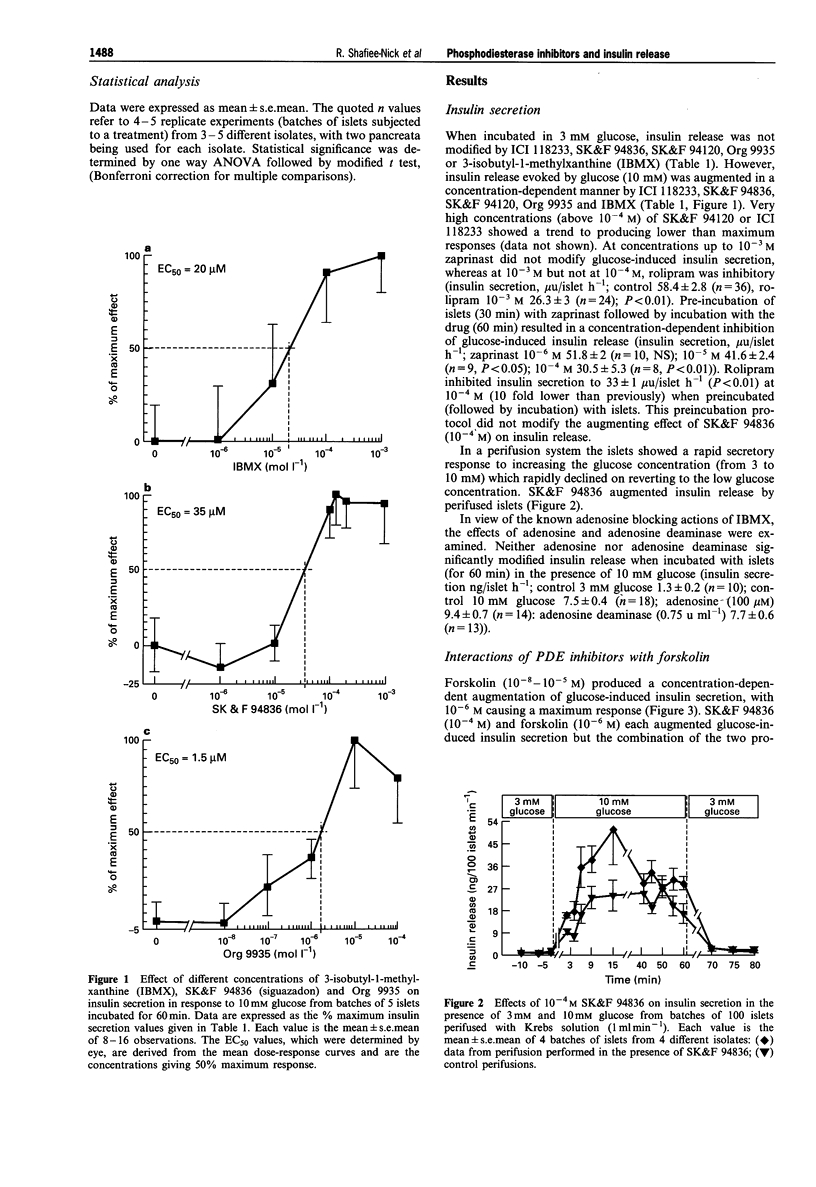
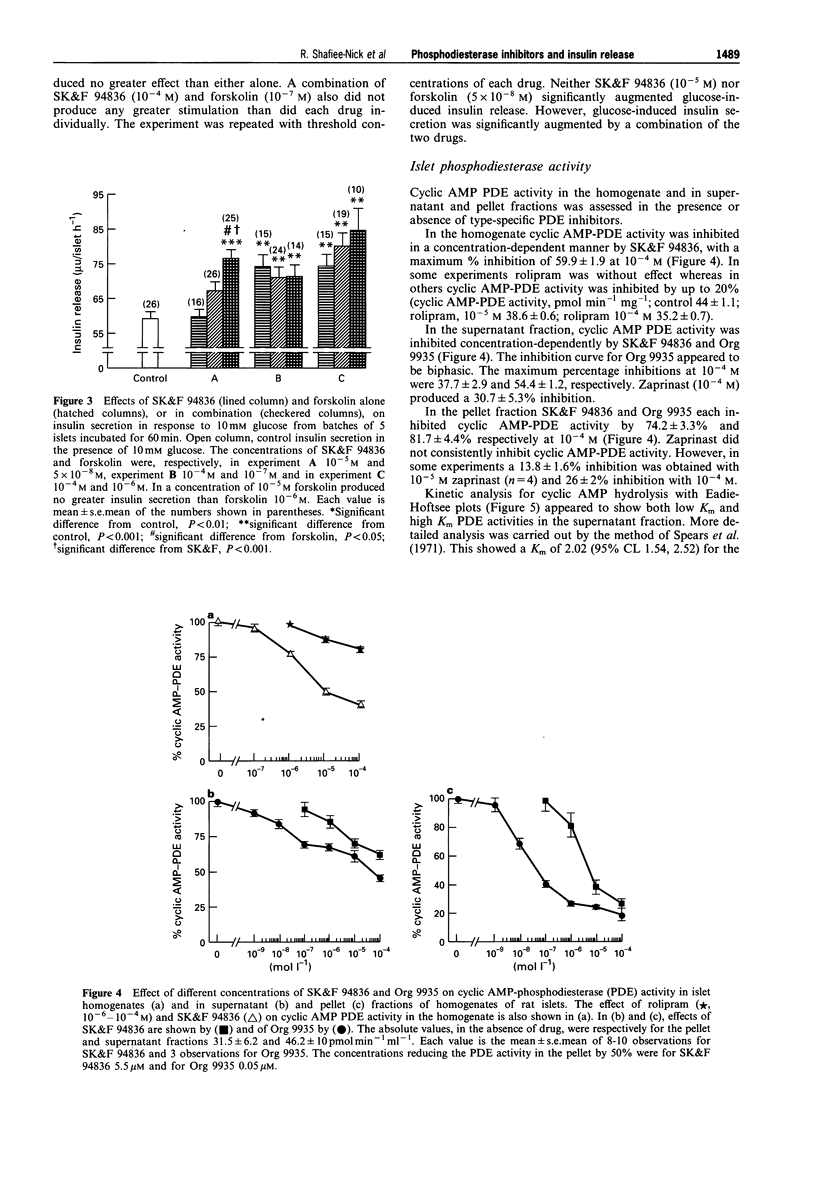
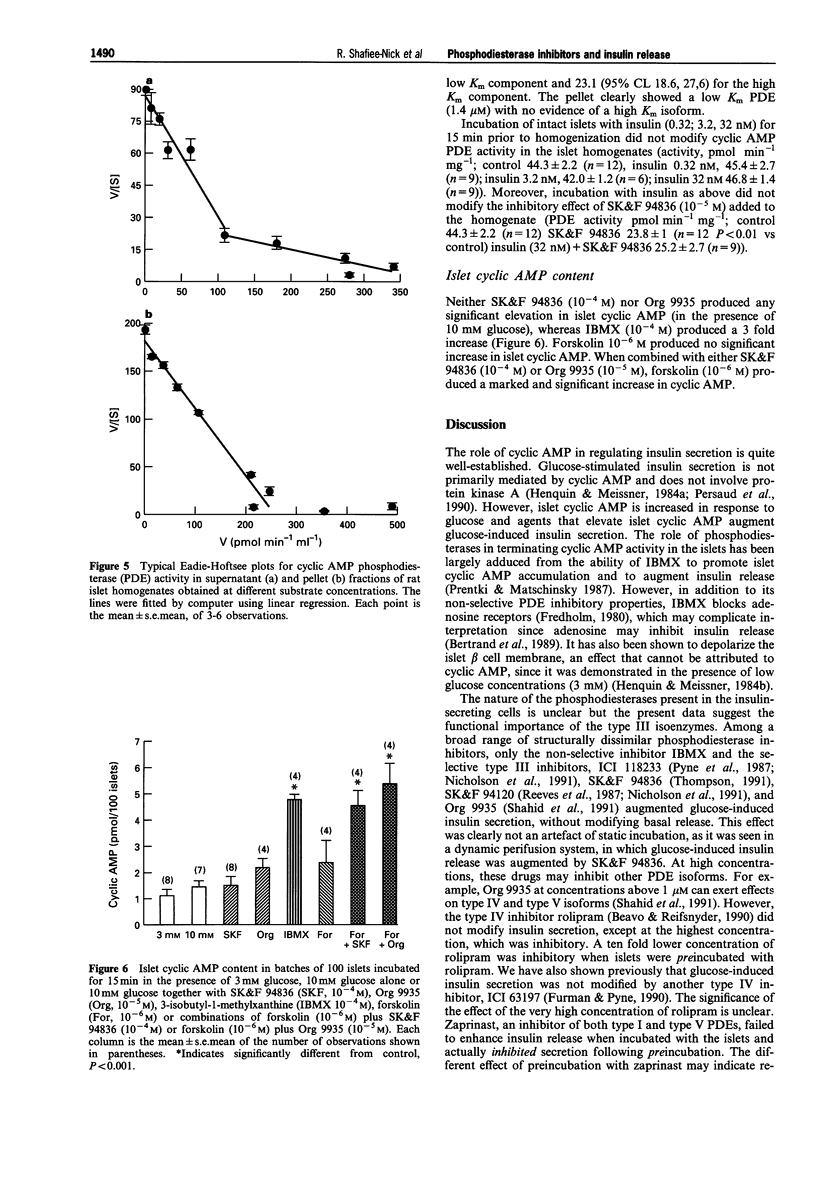
1. We examined various type-selective phosphodiesterase (PDE) inhibitors on glucose-induced insulin secretion from rat isolated islets, on islet PDE activity and on islet cyclic AMP accumulation in order to assess the relationship between type-selective PDE inhibition and modification of insulin release. 2. The non-selective PDE inhibitor, 3-isobutyl-1-methylxanthine (IBMX, 10(-5)-10(-3) M), as well as the type III selective PDE inhibitors SK&F 94836 (10(-5)-10(-3) M), Org 9935 (10(-7)-10(-4) M), SK&F 94120 (10(-5)-10(-4) M) and ICI 118233 (10(-6)-10(-4) M) each caused concentration-dependent augmentation (up to 40% increase) of insulin release in the presence of a stimulatory glucose concentration (10 mM), but not in the presence of 3 mM glucose. 3. Neither the type IV PDE inhibitor rolipram (10(-4) M) nor the type I and type V PDE inhibitor, zaprinast (10(-4)-10(-3) M) modified glucose-induced insulin release when incubated with islets, although a higher concentration of rolipram (10(-3) M) inhibited secretion by 55%. However, when islets were preincubated with these drugs followed by incubation in their continued presence, zaprinast (10(-6)-10(-4) M) produced a concentration-dependent inhibition (up to 45% at 10(-4) M). Under these conditions, rolipram inhibited insulin secretion at a lower concentration (10(-4) M) than when simply incubated with islets. 4. A combination of SK&F 94836 (10(-5) M) and forskolin (5 x 10(-8) M) significantly augmented glucose-induced insulin secretion (30% increase), although neither drug alone, in these concentrations, produced any significant effect.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Conti M., Heaslip R. J. Multiple cyclic nucleotide phosphodiesterases. Mol Pharmacol. 1994 Sep;46(3):399–405. [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bertrand G., Petit P., Bozem M., Henquin J. C. Membrane and intracellular effects of adenosine in mouse pancreatic beta-cells. Am J Physiol. 1989 Oct;257(4 Pt 1):E473–E478. doi: 10.1152/ajpendo.1989.257.4.E473. [DOI] [PubMed] [Google Scholar]
- Capito K., Hedeskov C. J., Thams P. Cyclic AMP phosphodiesterase activity in mouse pancreatic islets. Effects of calmodulin and phospholipids. Acta Endocrinol (Copenh) 1986 Apr;111(4):533–538. doi: 10.1530/acta.0.1110533. [DOI] [PubMed] [Google Scholar]
- Green I. C., Delaney C. A., Cunningham J. M., Karmiris V., Southern C. Interleukin-1 beta effects on cyclic GMP and cyclic AMP in cultured rat islets of Langerhans-arginine-dependence and relationship to insulin secretion. Diabetologia. 1993 Jan;36(1):9–16. doi: 10.1007/BF00399087. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Effects of theophylline and dibutyryl cyclic adenosine monophosphate on the membrane potential of mouse pancreatic beta-cells. J Physiol. 1984 Jun;351:595–612. doi: 10.1113/jphysiol.1984.sp015265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. The ionic, electrical, and secretory effects of endogenous cyclic adenosine monophosphate in mouse pancreatic B cells: studies with forskolin. Endocrinology. 1984 Sep;115(3):1125–1134. doi: 10.1210/endo-115-3-1125. [DOI] [PubMed] [Google Scholar]
- Holz G. G., Habener J. F. Signal transduction crosstalk in the endocrine system: pancreatic beta-cells and the glucose competence concept. Trends Biochem Sci. 1992 Oct;17(10):388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Bjaaland T., Pearson J. D., Howell S. L. Nitric oxide is not involved in the initiation of insulin secretion from rat islets of Langerhans. Diabetologia. 1992 Nov;35(11):1020–1027. doi: 10.1007/BF02221676. [DOI] [PubMed] [Google Scholar]
- Kelso E. J., McDermott B. J., Silke B. Cardiotonic actions of selective phosphodiesterase inhibitors in rat isolated ventricular cardiomyocytes. Br J Pharmacol. 1993 Dec;110(4):1387–1394. doi: 10.1111/j.1476-5381.1993.tb13974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Leibowitz M. D., Biswas C., Brady E. J., Conti M., Cullinan C. A., Hayes N. S., Manganiello V. C., Saperstein R., Wang L. H., Zafian P. T. A novel insulin secretagogue is a phosphodiesterase inhibitor. Diabetes. 1995 Jan;44(1):67–74. doi: 10.2337/diab.44.1.67. [DOI] [PubMed] [Google Scholar]
- Lipson L. G., Oldham S. B. The role of calmodulin in insulin secretion: the presence of a calmodulin-stimulatable phosphodiesterase in pancreatic islets of normal and pregnant rats. Life Sci. 1983 Feb 14;32(7):775–780. doi: 10.1016/0024-3205(83)90312-0. [DOI] [PubMed] [Google Scholar]
- Nicholson C. D., Challiss R. A., Shahid M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol Sci. 1991 Jan;12(1):19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- Persaud S. J., Jones P. M., Howell S. L. Glucose-stimulated insulin secretion is not dependent on activation of protein kinase A. Biochem Biophys Res Commun. 1990 Dec 31;173(3):833–839. doi: 10.1016/s0006-291x(05)80862-9. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Pyne N. J., Anderson N., Lavan B. E., Milligan G., Nimmo H. G., Houslay M. D. Specific antibodies and the selective inhibitor ICI 118233 demonstrate that the hormonally stimulated 'dense-vesicle' and peripheral-plasma-membrane cyclic AMP phosphodiesterases display distinct tissue distributions in the rat. Biochem J. 1987 Dec 15;248(3):897–901. doi: 10.1042/bj2480897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn T., Ridderstråle M., Tornqvist H., Manganiello V., Fredrikson G., Belfrage P., Degerman E. Essential role of phosphatidylinositol 3-kinase in insulin-induced activation and phosphorylation of the cGMP-inhibited cAMP phosphodiesterase in rat adipocytes. Studies using the selective inhibitor wortmannin. FEBS Lett. 1994 Aug 22;350(2-3):314–318. doi: 10.1016/0014-5793(94)00797-7. [DOI] [PubMed] [Google Scholar]
- Reeves M. L., Leigh B. K., England P. J. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987 Jan 15;241(2):535–541. doi: 10.1042/bj2410535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Cyclic AMP-dependent protein kinase from bovine heart muscle. Methods Enzymol. 1974;38:308–315. doi: 10.1016/0076-6879(74)38047-0. [DOI] [PubMed] [Google Scholar]
- Schuit F. C., Pipeleers D. G. Regulation of adenosine 3',5'-monophosphate levels in the pancreatic B cell. Endocrinology. 1985 Sep;117(3):834–840. doi: 10.1210/endo-117-3-834. [DOI] [PubMed] [Google Scholar]
- Shahid M., van Amsterdam R. G., de Boer J., ten Berge R. E., Nicholson C. D., Zaagsma J. The presence of five cyclic nucleotide phosphodiesterase isoenzyme activities in bovine tracheal smooth muscle and the functional effects of selective inhibitors. Br J Pharmacol. 1991 Oct;104(2):471–477. doi: 10.1111/j.1476-5381.1991.tb12453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears G., Sneyd J. G., Loten E. G. A method for deriving kinetic constants for two enzymes acting on the same substrate. Biochem J. 1971 Dec;125(4):1149–1151. doi: 10.1042/bj1251149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Ashcroft S. J. Cyclic nucleotide phosphodiesterase of rat pancreatic islets. Effects of Ca2+, calmodulin and trifluoperazine. Biochem J. 1981 Aug 1;197(2):459–464. doi: 10.1042/bj1970459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Thompson W. J. Cyclic nucleotide phosphodiesterases: pharmacology, biochemistry and function. Pharmacol Ther. 1991;51(1):13–33. doi: 10.1016/0163-7258(91)90039-o. [DOI] [PubMed] [Google Scholar]
- Vara E., Tamarit-Rodriguez J. Does cyclic guanosine monophosphate mediate noradrenaline-induced inhibition of islet insulin secretion stimulated by glucose and palmitate? Biochem J. 1991 Aug 15;278(Pt 1):243–248. doi: 10.1042/bj2780243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspohl E. J., Ammon H. P. Atrial natriuretic peptide (ANP) acts via specific binding sites on cGMP system of rat pancreatic islets without affecting insulin release. Naunyn Schmiedebergs Arch Pharmacol. 1989 Mar;339(3):348–353. doi: 10.1007/BF00173590. [DOI] [PubMed] [Google Scholar]
- Zawalich W. S., Rasmussen H. Control of insulin secretion: a model involving Ca2+, cAMP and diacylglycerol. Mol Cell Endocrinol. 1990 Apr 17;70(2):119–137. doi: 10.1016/0303-7207(90)90152-x. [DOI] [PubMed] [Google Scholar]


