Abstract
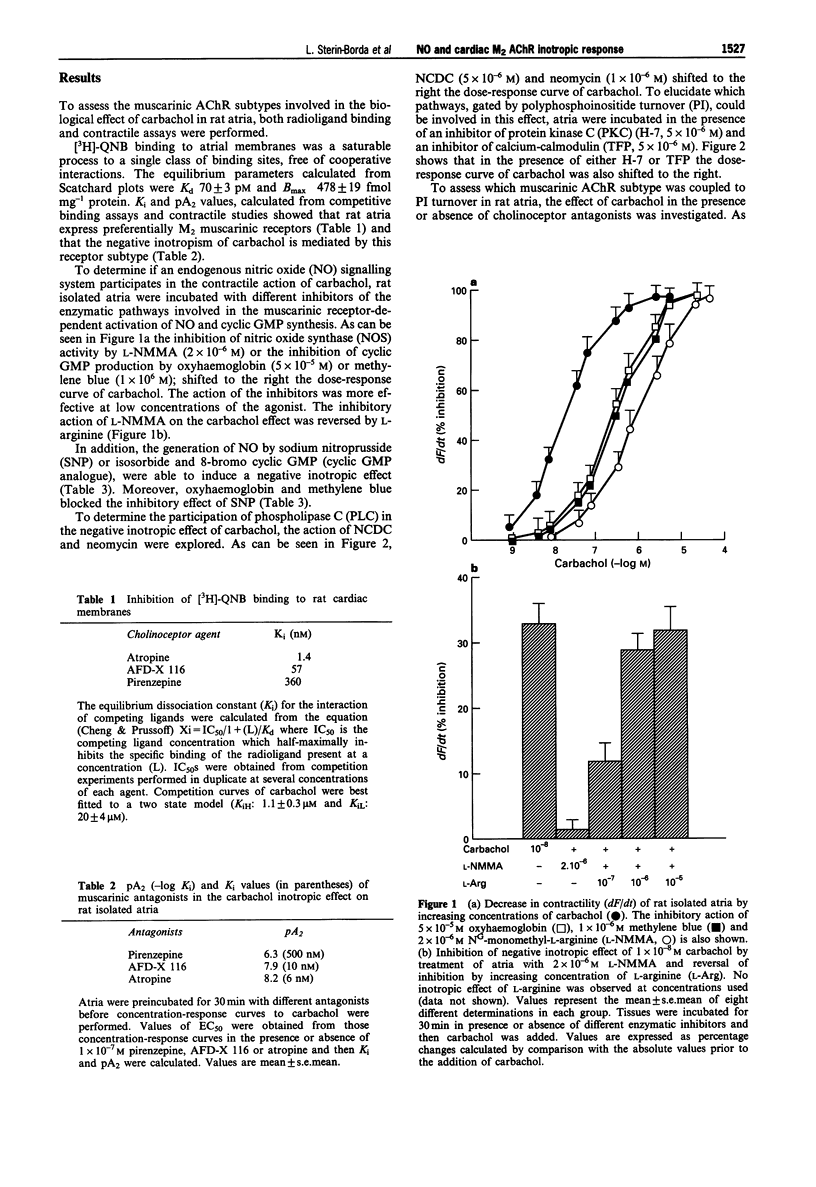
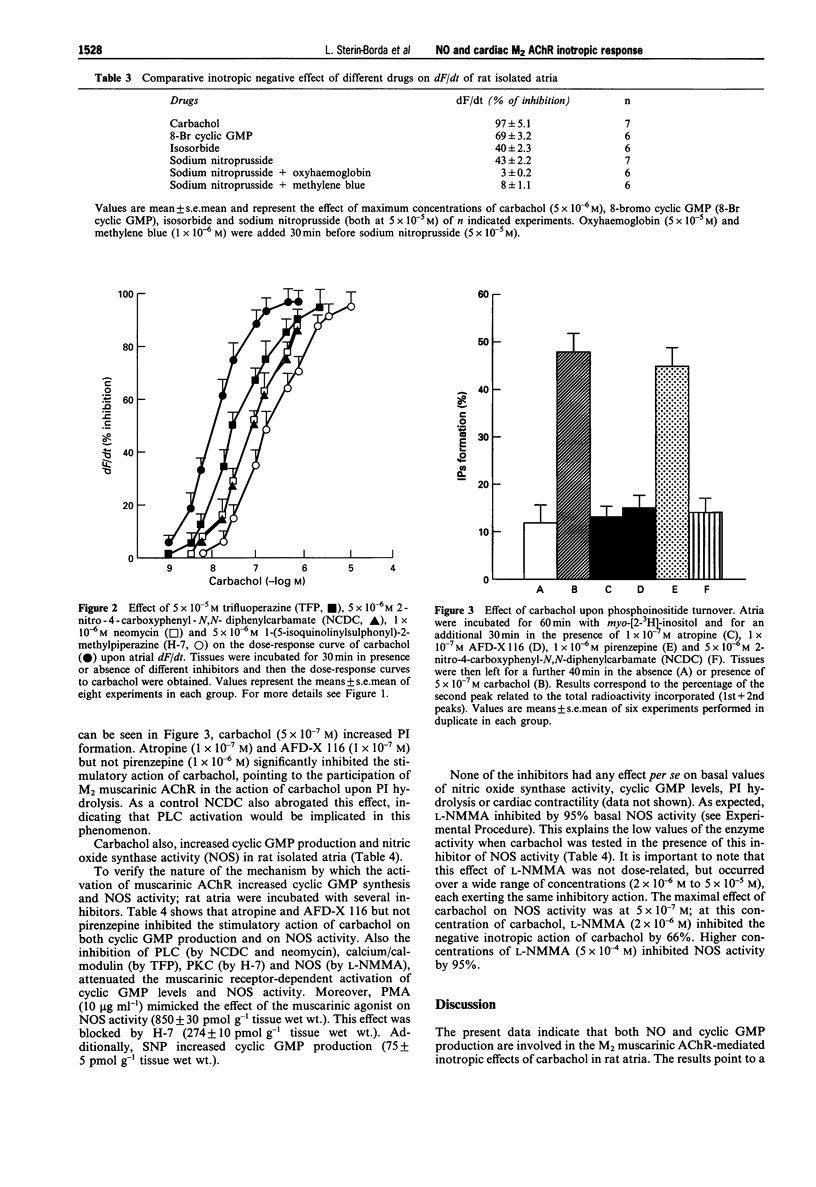
1. In this paper we have determined the different signalling pathways involved in muscarinic acetylcholine receptor (AChR)-dependent inhibition of contractility in rat isolated atria. 2. Carbachol stimulation of M2 muscarinic AChRs exerts a negative inotropic response, activation of phosphoinositide turnover, stimulation of nitric oxide synthase and increased production of cyclic GMP. 3. Inhibitors of phospholipase C, protein kinase C, calcium/calmodulin, nitric oxide synthase and guanylate cyclase, shifted the dose-response curve of carbachol on contractility to the right. These inhibitors also attenuated the muscarinic receptor-dependent increase in cyclic GMP and activation of nitric oxide synthase. In addition, sodium nitroprusside, isosorbide, or 8-bromo cyclic GMP, induced a negative inotropic effect, increased cyclic GMP and activated nitric oxide synthase. 4. These results suggest that carbachol activation of M2 AChRs, exerts a negative inotropic effect associated with increased production of nitric oxide and cyclic GMP. The mechanism appears to occur secondarily to stimulation of phosphoinositides turnover via phospholipase C activation. This in turn, triggers cascade reactions involving calcium/calmodulin and protein kinase C, leading to activation of nitric oxide synthase and soluble guanylate cyclase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Bacman S., Sterin-Borda L., Lustig L., Denduchis B., Borda E. Antilaminin IgG binds and interacts with cardiac cholinergic receptors. Can J Physiol Pharmacol. 1990 Apr;68(4):539–544. doi: 10.1139/y90-078. [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda E., Pascual J., Cossio P., De La Vega M., Arana R., Sterin-Borda L. A circulating IgG in Chagas' disease which binds to beta-adrenoceptors of myocardium and modulates their activity. Clin Exp Immunol. 1984 Sep;57(3):679–686. [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Brown S. L. Agonists differentiate muscarinic receptors that inhibit cyclic AMP formation from those that stimulate phosphoinositide metabolism. J Biol Chem. 1984 Mar 25;259(6):3777–3781. [PubMed] [Google Scholar]
- Brown J. H., Buxton I. L., Brunton L. L. Alpha 1-adrenergic and muscarinic cholinergic stimulation of phosphoinositide hydrolysis in adult rat cardiomyocytes. Circ Res. 1985 Oct;57(4):532–537. doi: 10.1161/01.res.57.4.532. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Goldstein D., Masters S. B. The putative M1 muscarinic receptor does not regulate phosphoinositide hydrolysis. Studies with pirenzepine and McN-A343 in chick heart and astrocytoma cells. Mol Pharmacol. 1985 May;27(5):525–531. [PubMed] [Google Scholar]
- Buxton I. L., Cheek D. J., Eckman D., Westfall D. P., Sanders K. M., Keef K. D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993 Feb;72(2):387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Candell L. M., Yun S. H., Tran L. L., Ehlert F. J. Differential coupling of subtypes of the muscarinic receptor to adenylate cyclase and phosphoinositide hydrolysis in the longitudinal muscle of the rat ileum. Mol Pharmacol. 1990 Nov;38(5):689–697. [PubMed] [Google Scholar]
- Entzeroth M., Doods H. N., Mayer N. Characterization of porcine coronary muscarinic receptors. Naunyn Schmiedebergs Arch Pharmacol. 1990 May;341(5):432–438. doi: 10.1007/BF00176336. [DOI] [PubMed] [Google Scholar]
- Feigenbaum P., El-Fakahany E. E. Regulation of muscarinic cholinergic receptor density in neuroblastoma cells by brief exposure to agonist: possible involvement in desensitization of receptor function. J Pharmacol Exp Ther. 1985 Apr;233(1):134–140. [PubMed] [Google Scholar]
- Förstermann U., Gorsky L. D., Pollock J. S., Ishii K., Schmidt H. H., Heller M., Murad F. Hormone-induced biosynthesis of endothelium-derived relaxing factor/nitric oxide-like material in N1E-115 neuroblastoma cells requires calcium and calmodulin. Mol Pharmacol. 1990 Jul;38(1):7–13. [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goin J. C., Borda E., Leiros C. P., Storino R., Sterin-Borda L. Identification of antibodies with muscarinic cholinergic activity in human Chagas' disease: pathological implications. J Auton Nerv Syst. 1994 Apr;47(1-2):45–52. doi: 10.1016/0165-1838(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Gold M. E., Bush P. A., Ignarro L. J. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Oct 31;164(2):714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- Hokin-Neaverson M., Sadeghian K. Separation of [3H] inositol monophosphates and [3H] inositol on silica gel glass-fiber sheets. J Chromatogr. 1976 May 26;120(2):502–505. doi: 10.1016/s0021-9673(76)80031-3. [DOI] [PubMed] [Google Scholar]
- Hortelano S., Genaro A. M., Boscá L. Phorbol esters induce nitric oxide synthase activity in rat hepatocytes. Antagonism with the induction elicited by lipopolysaccharide. J Biol Chem. 1992 Dec 15;267(35):24937–24940. [PubMed] [Google Scholar]
- Hortelano S., Genaro A. M., Boscá L. Phorbol esters induce nitric oxide synthase and increase arginine influx in cultured peritoneal macrophages. FEBS Lett. 1993 Apr 5;320(2):135–139. doi: 10.1016/0014-5793(93)80078-9. [DOI] [PubMed] [Google Scholar]
- Hosey M. M. Diversity of structure, signaling and regulation within the family of muscarinic cholinergic receptors. FASEB J. 1992 Feb 1;6(3):845–852. [PubMed] [Google Scholar]
- Ikegaya T., Nishiyama T., Haga K., Haga T., Ichiyama A., Kobayashi A., Yamazaki N. Interaction of atrial muscarinic receptors with three kinds of GTP-binding proteins. J Mol Cell Cardiol. 1990 Mar;22(3):343–351. doi: 10.1016/0022-2828(90)91467-l. [DOI] [PubMed] [Google Scholar]
- Lai W. S., el-Fakahany E. E. Phorbol ester-induced inhibition of cyclic GMP formation mediated by muscarinic receptors in murine neuroblastoma cells. J Pharmacol Exp Ther. 1987 May;241(2):366–373. [PubMed] [Google Scholar]
- Liles W. C., Hunter D. D., Meier K. E., Nathanson N. M. Activation of protein kinase C induces rapid internalization and subsequent degradation of muscarinic acetylcholine receptors in neuroblastoma cells. J Biol Chem. 1986 Apr 25;261(12):5307–5313. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Méry P. F., Lohmann S. M., Walter U., Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Nakane M., Mitchell J., Förstermann U., Murad F. Phosphorylation by calcium calmodulin-dependent protein kinase II and protein kinase C modulates the activity of nitric oxide synthase. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1396–1402. doi: 10.1016/s0006-291x(05)81351-8. [DOI] [PubMed] [Google Scholar]
- Peterson G. L., Herron G. S., Yamaki M., Fullerton D. S., Schimerlik M. I. Purification of the muscarinic acetylcholine receptor from porcine atria. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4993–4997. doi: 10.1073/pnas.81.15.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Nava E., Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992 Mar;105(3):575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. M., Lewis M. J., Henderson A. H. Effects of 8-bromo-cyclic GMP on contraction and on inotropic response of ferret cardiac muscle. J Mol Cell Cardiol. 1991 Jan;23(1):55–64. doi: 10.1016/0022-2828(91)90038-n. [DOI] [PubMed] [Google Scholar]
- Stein B., Drögemüller A., Mülsch A., Schmitz W., Scholz H. Ca(++)-dependent constitutive nitric oxide synthase is not involved in the cyclic GMP-increasing effects of carbachol in ventricular cardiomyocytes. J Pharmacol Exp Ther. 1993 Aug;266(2):919–925. [PubMed] [Google Scholar]
- Sterin-Borda L., Cantore M., Pascual J., Borda E., Cossio P., Arana R., Passeron S. Chagasic IgG binds and interacts with cardiac beta adrenoceptor-coupled adenylate cyclase system. Int J Immunopharmacol. 1986;8(6):581–588. doi: 10.1016/0192-0561(86)90029-9. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Taniguchi J., Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982 Feb;392(4):307–314. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J. M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Wood K. S., Buga G. M., Byrns R. E., Ignarro L. J. Vascular smooth muscle-derived relaxing factor (MDRF) and its close similarity to nitric oxide. Biochem Biophys Res Commun. 1990 Jul 16;170(1):80–88. doi: 10.1016/0006-291x(90)91243-l. [DOI] [PubMed] [Google Scholar]