Abstract
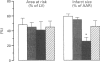
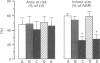
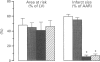
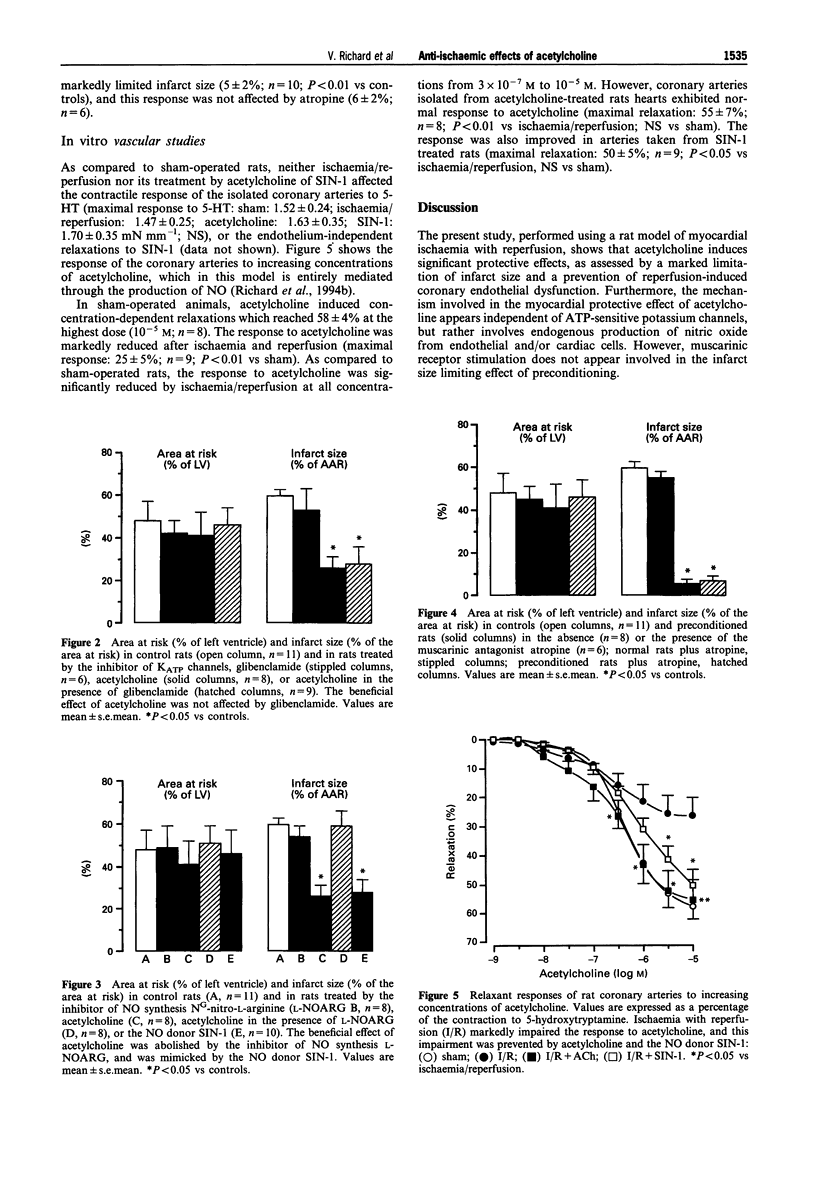
1. Recent experiments suggest that acetylcholine (ACh) may exert myocardial protective effects during ischaemia (I) and reperfusion (R). The present study was designed (i) to assess whether ACh limits infarct size and protects coronary endothelial cells in a rat model of I and R, (ii) to evaluate the role of ATP-sensitive potassium (KATP) channels and nitric oxide (NO) in the beneficial effect of ACh (iii) to evaluate whether the protective effect of ACh also extends to coronary endothelial cells and (iv) to assess whether ACh contributes to the beneficial effect of preconditioning. 2. Anaesthetized rats were subjected to 20 min I (left coronary artery occlusion) and 2 h of R. Infarct size was assessed by triphenyltetrazolium (TTC) staining and expressed as a % of the area at risk (India ink injection). Vascular studies were performed on 1.5-2 mm coronary segments (internal diameter 250-300 micros) removed distal to the site of occlusion and mounted in wire myographs. 3. ACh limited infarct size (from 59 +/- 3 to 26 +/- 5%, P < 0.01), and this was prevented by atropine (46 +/- 7%; P < 0.05 vs ACh), but not by the inhibitor of KATP channels, glibenclamide (29 +/- 8%). The inhibitor of NO synthesis NG-nitro L-arginine did not affect infarct size (54 +/- 5%) but abolished the beneficial effect of ACh (59 +/- 8%; P < 0.05 vs ACh), whereas the NO donor 3-morpholinosydnonimine-N-ethylcarbamide (SIN-1 limited infarct size to the same extent as ACh (28 +/- 6%).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchampach J. A., Maruyama M., Cavero I., Gross G. J. The new K+ channel opener Aprikalim (RP 52891) reduces experimental infarct size in dogs in the absence of hemodynamic changes. J Pharmacol Exp Ther. 1991 Dec;259(3):961–967. [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Locke-Winter C., Rogers K. B., Mitchell M. B., Brew E. C., Cairns C. B., Bensard D. D., Harken A. H. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an alpha 1-adrenergic mechanism. Circ Res. 1993 Oct;73(4):656–670. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady A. J., Warren J. B., Poole-Wilson P. A., Williams T. J., Harding S. E. Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol. 1993 Jul;265(1 Pt 2):H176–H182. doi: 10.1152/ajpheart.1993.265.1.H176. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Cheek D. J., Eckman D., Westfall D. P., Sanders K. M., Keef K. D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993 Feb;72(2):387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Leszczynska-Piziak J., Abramson S. B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992 Sep;90(3):1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocott-Mason R., Fort S., Lewis M. J., Shah A. M. Myocardial relaxant effect of exogenous nitric oxide in isolated ejecting hearts. Am J Physiol. 1994 May;266(5 Pt 2):H1699–H1705. doi: 10.1152/ajpheart.1994.266.5.H1699. [DOI] [PubMed] [Google Scholar]
- Gross G. J., Auchampach J. A. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992 Feb;70(2):223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- Grover G. J., McCullough J. R., Henry D. E., Conder M. L., Sleph P. G. Anti-ischemic effects of the potassium channel activators pinacidil and cromakalim and the reversal of these effects with the potassium channel blocker glyburide. J Pharmacol Exp Ther. 1989 Oct;251(1):98–104. [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Bailie M. B., Abrams G. D., Lucchesi B. R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984 Mar;54(3):277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L., Kummer W., Mayer B., Couraud J. Y., Preissler U., Philippin B., Heym C. Nitric oxide synthase in cardiac nerve fibers and neurons of rat and guinea pig heart. Circ Res. 1992 Dec;71(6):1533–1537. doi: 10.1161/01.res.71.6.1533. [DOI] [PubMed] [Google Scholar]
- Ku D. D. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982 Nov 5;218(4572):576–578. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer D. J., Nakanishi K., Johnston W. E., Vinten-Johansen J. Antineutrophil and myocardial protecting actions of a novel nitric oxide donor after acute myocardial ischemia and reperfusion of dogs. Circulation. 1993 Nov;88(5 Pt 1):2337–2350. doi: 10.1161/01.cir.88.5.2337. [DOI] [PubMed] [Google Scholar]
- Li Y., Kloner R. A. Does protein kinase C play a role in ischemic preconditioning in rat hearts? Am J Physiol. 1995 Jan;268(1 Pt 2):H426–H431. doi: 10.1152/ajpheart.1995.268.1.H426. [DOI] [PubMed] [Google Scholar]
- Li Y., Kloner R. A. The cardioprotective effects of ischemic 'preconditioning' are not mediated by adenosine receptors in rat hearts. Circulation. 1993 May;87(5):1642–1648. doi: 10.1161/01.cir.87.5.1642. [DOI] [PubMed] [Google Scholar]
- Liu Y., Downey J. M. Ischemic preconditioning protects against infarction in rat heart. Am J Physiol. 1992 Oct;263(4 Pt 2):H1107–H1112. doi: 10.1152/ajpheart.1992.263.4.H1107. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Weyrich A. S., Lefer D. J., Lefer A. M. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993 Feb;72(2):403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- Matheis G., Sherman M. P., Buckberg G. D., Haybron D. M., Young H. H., Ignarro L. J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am J Physiol. 1992 Feb;262(2 Pt 2):H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- Mitchell M. B., Meng X., Ao L., Brown J. M., Harken A. H., Banerjee A. Preconditioning of isolated rat heart is mediated by protein kinase C. Circ Res. 1995 Jan;76(1):73–81. doi: 10.1161/01.res.76.1.73. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Vinten-Johansen J., Lefer D. J., Zhao Z., Fowler W. C., 3rd, McGee D. S., Johnston W. E. Intracoronary L-arginine during reperfusion improves endothelial function and reduces infarct size. Am J Physiol. 1992 Dec;263(6 Pt 2):H1650–H1658. doi: 10.1152/ajpheart.1992.263.6.H1650. [DOI] [PubMed] [Google Scholar]
- Patel V. C., Yellon D. M., Singh K. J., Neild G. H., Woolfson R. G. Inhibition of nitric oxide limits infarct size in the in situ rabbit heart. Biochem Biophys Res Commun. 1993 Jul 15;194(1):234–238. doi: 10.1006/bbrc.1993.1809. [DOI] [PubMed] [Google Scholar]
- Pearson P. J., Schaff H. V., Vanhoutte P. M. Acute impairment of endothelium-dependent relaxations to aggregating platelets following reperfusion injury in canine coronary arteries. Circ Res. 1990 Aug;67(2):385–393. doi: 10.1161/01.res.67.2.385. [DOI] [PubMed] [Google Scholar]
- Pearson P. J., Schaff H. V., Vanhoutte P. M. Long-term impairment of endothelium-dependent relaxations to aggregating platelets after reperfusion injury in canine coronary arteries. Circulation. 1990 Jun;81(6):1921–1927. doi: 10.1161/01.cir.81.6.1921. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Reimer K. A., Murry C. E., Richard V. J. The role of neutrophils and free radicals in the ischemic-reperfused heart: why the confusion and controversy? J Mol Cell Cardiol. 1989 Dec;21(12):1225–1239. doi: 10.1016/0022-2828(89)90669-x. [DOI] [PubMed] [Google Scholar]
- Richard V. J., Murry C. E., Jennings R. B., Reimer K. A. Therapy to reduce free radicals during early reperfusion does not limit the size of myocardial infarcts caused by 90 minutes of ischemia in dogs. Circulation. 1988 Aug;78(2):473–480. doi: 10.1161/01.cir.78.2.473. [DOI] [PubMed] [Google Scholar]
- Richard V., Kaeffer N., Hogie M., Tron C., Blanc T., Thuillez C. Role of endogenous endothelin in myocardial and coronary endothelial injury after ischaemia and reperfusion in rats: studies with bosentan, a mixed ETA-ETB antagonist. Br J Pharmacol. 1994 Nov;113(3):869–876. doi: 10.1111/j.1476-5381.1994.tb17073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard V., Kaeffer N., Tron C., Thuillez C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation. 1994 Mar;89(3):1254–1261. doi: 10.1161/01.cir.89.3.1254. [DOI] [PubMed] [Google Scholar]
- Richard V., Tron C., Thuillez C. Ischaemic preconditioning is not mediated by oxygen derived free radicals in rats. Cardiovasc Res. 1993 Nov;27(11):2016–2021. doi: 10.1093/cvr/27.11.2016. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Ho E. H., Cantor E. H., Lumma W. C., Botelho L. H. Cytoprotective function of nitric oxide: inactivation of superoxide radicals produced by human leukocytes. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1392–1397. doi: 10.1016/0006-291x(91)92093-y. [DOI] [PubMed] [Google Scholar]
- Schulz R., Smith J. A., Lewis M. J., Moncada S. Nitric oxide synthase in cultured endocardial cells of the pig. Br J Pharmacol. 1991 Sep;104(1):21–24. doi: 10.1111/j.1476-5381.1991.tb12378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. M., Spurgeon H. A., Sollott S. J., Talo A., Lakatta E. G. 8-bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ Res. 1994 May;74(5):970–978. doi: 10.1161/01.res.74.5.970. [DOI] [PubMed] [Google Scholar]
- Siegfried M. R., Carey C., Ma X. L., Lefer A. M. Beneficial effects of SPM-5185, a cysteine-containing NO donor in myocardial ischemia-reperfusion. Am J Physiol. 1992 Sep;263(3 Pt 2):H771–H777. doi: 10.1152/ajpheart.1992.263.3.H771. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Fantone J. C., Mickelson J. K., Griffin J. D., Lucchesi B. R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988 Feb;81(2):624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Shah A. M., Lewis M. J. Factors released from endocardium of the ferret and pig modulate myocardial contraction. J Physiol. 1991 Aug;439:1–14. doi: 10.1113/jphysiol.1991.sp018653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speechly-Dick M. E., Mocanu M. M., Yellon D. M. Protein kinase C. Its role in ischemic preconditioning in the rat. Circ Res. 1994 Sep;75(3):586–590. doi: 10.1161/01.res.75.3.586. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Brooks S. E., Richard V. J., FitzHarris G. P., Stoler R. C., Jennings R. B., Arfors K. E., Reimer K. A. Effect of anti-CD18 antibody on myocardial neutrophil accumulation and infarct size after ischemia and reperfusion in dogs. Circulation. 1993 Feb;87(2):526–535. doi: 10.1161/01.cir.87.2.526. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Richard V. J., Murry C. E., Jennings R. B., Reimer K. A. Superoxide dismutase plus catalase therapy delays neither cell death nor the loss of the TTC reaction in experimental myocardial infarction in dogs. J Mol Cell Cardiol. 1993 Apr;25(4):367–378. doi: 10.1006/jmcc.1993.1043. [DOI] [PubMed] [Google Scholar]
- Thornton J. D., Liu G. S., Downey J. M. Pretreatment with pertussis toxin blocks the protective effects of preconditioning: evidence for a G-protein mechanism. J Mol Cell Cardiol. 1993 Mar;25(3):311–320. doi: 10.1006/jmcc.1993.1037. [DOI] [PubMed] [Google Scholar]
- Tsao P. S., Aoki N., Lefer D. J., Johnson G., 3rd, Lefer A. M. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation. 1990 Oct;82(4):1402–1412. doi: 10.1161/01.cir.82.4.1402. [DOI] [PubMed] [Google Scholar]
- VanBenthuysen K. M., McMurtry I. F., Horwitz L. D. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J Clin Invest. 1987 Jan;79(1):265–274. doi: 10.1172/JCI112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfson R. G., Patel V. C., Neild G. H., Yellon D. M. Inhibition of nitric oxide synthesis reduces infarct size by an adenosine-dependent mechanism. Circulation. 1995 Mar 1;91(5):1545–1551. doi: 10.1161/01.cir.91.5.1545. [DOI] [PubMed] [Google Scholar]
- Yao Z., Gross G. J. Acetylcholine mimics ischemic preconditioning via a glibenclamide-sensitive mechanism in dogs. Am J Physiol. 1993 Jun;264(6 Pt 2):H2221–H2225. doi: 10.1152/ajpheart.1993.264.6.H2221. [DOI] [PubMed] [Google Scholar]
- Yao Z., Gross G. J. Role of nitric oxide, muscarinic receptors, and the ATP-sensitive K+ channel in mediating the effects of acetylcholine to mimic preconditioning in dogs. Circ Res. 1993 Dec;73(6):1193–1201. doi: 10.1161/01.res.73.6.1193. [DOI] [PubMed] [Google Scholar]
- Yellon D. M., Alkhulaifi A. M., Browne E. E., Pugsley W. B. Ischaemic preconditioning limits infarct size in the rat heart. Cardiovasc Res. 1992 Oct;26(10):983–987. doi: 10.1093/cvr/26.10.983. [DOI] [PubMed] [Google Scholar]