Abstract
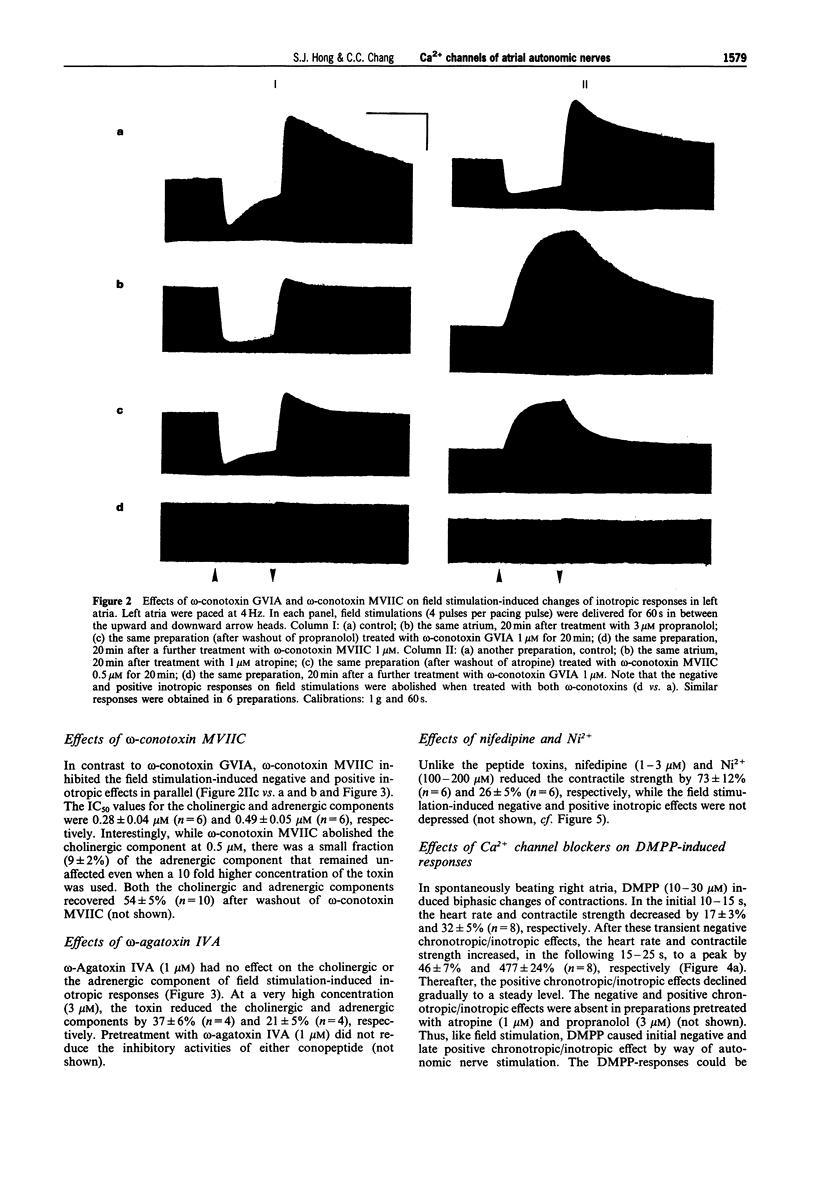
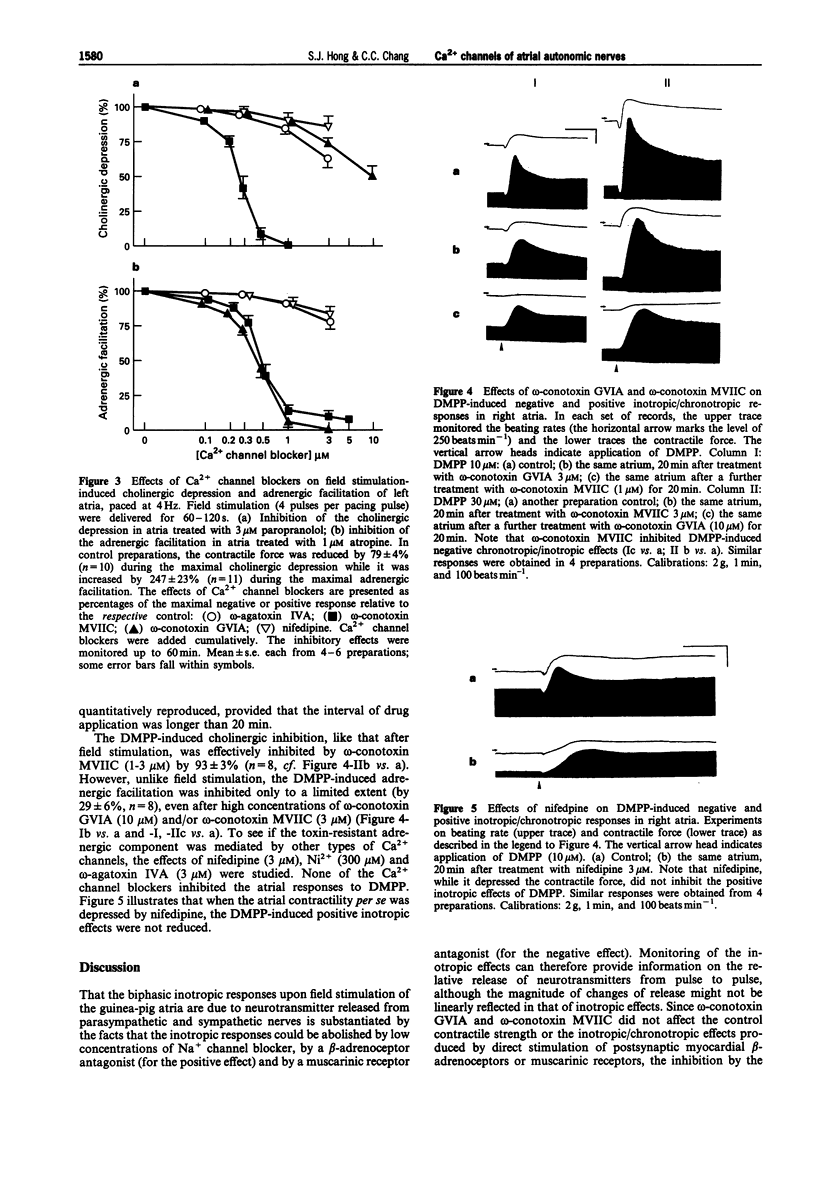
1. The Ca2+ channel subtypes of the autonomic nerves of guinea-pig atria were elucidated by monitoring the effects of specific Ca2+ channel blockers on the negative and positive inotropic responses associated respectively, with stimulation of the parasympathetic and sympathetic nerves. 2. In left atria paced at 2-4 Hz, the negative inotropic effect induced by field stimulation of parasympathetic nerves (in the presence of propranolol) was abolished by omega-conotoxin MVIIC, a blocker of N-type and OPQ subfamily Ca2+ channels. omega-Conotoxin GVIA (an N-type blocker), omega-agatoxin IVA (a P-type blocker), nifedipine (an L-type blocker) and Ni2+ (a T- and R-type blocker) were much less effective. 3. The positive inotropic response resulting from field stimulation of the sympathetic nerves (in the presence of atropine) was abolished by both omega-conotoxins, while omega-agatoxin IVA, nifedipine and Ni2+ were ineffective. 4. In the spontaneously beating right atria, the early negative inotropic effect produced by 1,1-dimethyl-4-phenylpiperazinium was abolished by omega-conotoxin MVIIC, whereas the late positive inotropic effect was partially reduced, but not abolished, by a high concentration of omega-conotoxin GVIA. 5. None of the peptide toxins affected the chronotropic and the inotropic responses evoked by carbachol and isoprenaline. 6. These results suggested that, under physiological conditions, the release of acetylcholine from parasympathetic nerves is dominated by an OPQ subfamily Ca2+ channel while that of noradrenaline from sympathetic nerves is controlled by an N-type Ca2+ channel. Ligand-induced noradrenaline release appeared to recruit additional type(s) of Ca2+ channel.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albillos A., Artalejo A. R., López M. G., Gandía L., García A. G., Carbone E. Calcium channel subtypes in cat chromaffin cells. J Physiol. 1994 Jun 1;477(Pt 2):197–213. doi: 10.1113/jphysiol.1994.sp020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A., Clarac F., Buño W. P-type Ca2+ channels mediate excitatory and inhibitory synaptic transmitter release in crayfish muscle. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4224–4228. doi: 10.1073/pnas.91.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Adams M. E., Fox A. P. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994 Jan 6;367(6458):72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- Bertolino M., Llinás R. R. The central role of voltage-activated and receptor-operated calcium channels in neuronal cells. Annu Rev Pharmacol Toxicol. 1992;32:399–421. doi: 10.1146/annurev.pa.32.040192.002151. [DOI] [PubMed] [Google Scholar]
- Blinks J. R. Field stimulation as a means of effecting the graded release of autonomic transmitters in isolated heart muscle. J Pharmacol Exp Ther. 1966 Feb;151(2):221–235. [PubMed] [Google Scholar]
- Boot J. R. Differential effects of omega-conotoxin GVIA and MVIIC on nerve stimulation induced contractions of guinea-pig ileum and rat vas deferens. Eur J Pharmacol. 1994 Jun 2;258(1-2):155–158. doi: 10.1016/0014-2999(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Cohen M. W., Jones O. T., Angelides K. J. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci. 1991 Apr;11(4):1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A., Li C. G., Rand M. J., Reid J. J., Thaina P., Wong-Dusting H. K. Effects of omega-conotoxin GVIA on autonomic neuroeffector transmission in various tissues. Br J Pharmacol. 1990 Oct;101(2):437–447. doi: 10.1111/j.1476-5381.1990.tb12727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Luebke J. I., Turner T. J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995 Feb;18(2):89–98. [PubMed] [Google Scholar]
- Ellinor P. T., Zhang J. F., Randall A. D., Zhou M., Schwarz T. L., Tsien R. W., Horne W. A. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993 Jun 3;363(6428):455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Fariñas I., Egea G., Blasi J., Cases C., Marsal J. Calcium channel antagonist omega-conotoxin binds to intramembrane particles of isolated nerve terminals. Neuroscience. 1993 Jun;54(3):745–752. doi: 10.1016/0306-4522(93)90244-a. [DOI] [PubMed] [Google Scholar]
- Fossier P., Baux G., Tauc L. N- and P-type Ca2+ channels are involved in acetylcholine release at a neuroneuronal synapse: only the N-type channel is the target of neuromodulators. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4771–4775. doi: 10.1073/pnas.91.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Burgos G. R., Biali F. I., Cherksey B. D., Sugimori M., Llinás R. R., Uchitel O. D. Different calcium channels mediate transmitter release evoked by transient or sustained depolarization at mammalian sympathetic ganglia. Neuroscience. 1995 Jan;64(1):117–123. doi: 10.1016/0306-4522(94)00368-f. [DOI] [PubMed] [Google Scholar]
- Grantham C. J., Bowman D., Bath C. P., Bell D. C., Bleakman D. Omega-conotoxin MVIIC reversibly inhibits a human N-type calcium channel and calcium influx into chick synaptosomes. Neuropharmacology. 1994 Feb;33(2):255–258. doi: 10.1016/0028-3908(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hillyard D. R., Monje V. D., Mintz I. M., Bean B. P., Nadasdi L., Ramachandran J., Miljanich G., Azimi-Zoonooz A., McIntosh J. M., Cruz L. J. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992 Jul;9(1):69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Biel M., Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Inhibition of acetylcholine release from mouse motor nerve by a P-type calcium channel blocker, omega-agatoxin IVA. J Physiol. 1995 Jan 15;482(Pt 2):283–290. doi: 10.1113/jphysiol.1995.sp020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Lindmar R., Löffelholz K., Muscholl E. A muscarinic mechanism inhibiting the release of noradrenaline from peripheral adrenergic nerve fibres by nicotinic agents. Br J Pharmacol Chemother. 1968 Feb;32(2):280–294. doi: 10.1111/j.1476-5381.1968.tb00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- López M. G., Albillos A., de la Fuente M. T., Borges R., Gandía L., Carbone E., García A. G., Artalejo A. R. Localized L-type calcium channels control exocytosis in cat chromaffin cells. Pflugers Arch. 1994 Jun;427(3-4):348–354. doi: 10.1007/BF00374544. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Momiyama A., Takahashi T. Calcium channels responsible for potassium-induced transmitter release at rat cerebellar synapses. J Physiol. 1994 Apr 15;476(2):197–202. doi: 10.1113/jphysiol.1994.sp020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Gray W. R., Zeikus R., McIntosh J. M., Varga J., Rivier J., de Santos V., Cruz L. J. Peptide neurotoxins from fish-hunting cone snails. Science. 1985 Dec 20;230(4732):1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Miljanich G. P., Ramachandran J., Adams M. E. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Protti D. A., Uchitel O. D. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993 Dec 13;5(3):333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- Pruneau D., Angus J. A. Apparent vascular to cardiac sympatholytic selectivity of omega-conotoxin GVIA in the pithed rat. Eur J Pharmacol. 1990 Aug 2;184(1):127–133. doi: 10.1016/0014-2999(90)90673-t. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Mintz I. M. Participation of multiple calcium channel types in transmission at single climbing fiber to Purkinje cell synapses. Neuron. 1994 Mar;12(3):605–613. doi: 10.1016/0896-6273(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Sather W. A., Tanabe T., Zhang J. F., Mori Y., Adams M. E., Tsien R. W. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel alpha 1 subunits. Neuron. 1993 Aug;11(2):291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Adams M. E., Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel O. D., Protti D. A., Sanchez V., Cherksey B. D., Sugimori M., Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I., Dooley D. J., Werhand J., Schlemmer F. Differential effects of calcium channel antagonists (omega-conotoxin GVIA, nifedipine, verapamil) on the electrically-evoked release of [3H]acetylcholine from the myenteric plexus, phrenic nerve and neocortex of rats. Naunyn Schmiedebergs Arch Pharmacol. 1990 Apr;341(4):288–294. doi: 10.1007/BF00180653. [DOI] [PubMed] [Google Scholar]
- Westfall T. C., Brasted M. The mechanism of action of nicotine on adrenergic neurons in the perfused guinea-pig heart. J Pharmacol Exp Ther. 1972 Sep;182(3):409–418. [PubMed] [Google Scholar]
- Wheeler D. B., Randall A., Tsien R. W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994 Apr 1;264(5155):107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Yawo H., Chuhma N. Preferential inhibition of omega-conotoxin-sensitive presynaptic Ca2+ channels by adenosine autoreceptors. Nature. 1993 Sep 16;365(6443):256–258. doi: 10.1038/365256a0. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993 Nov;32(11):1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Calcium and transmitter release. J Physiol Paris. 1993;87(1):25–36. doi: 10.1016/0928-4257(93)90021-k. [DOI] [PubMed] [Google Scholar]


