Abstract
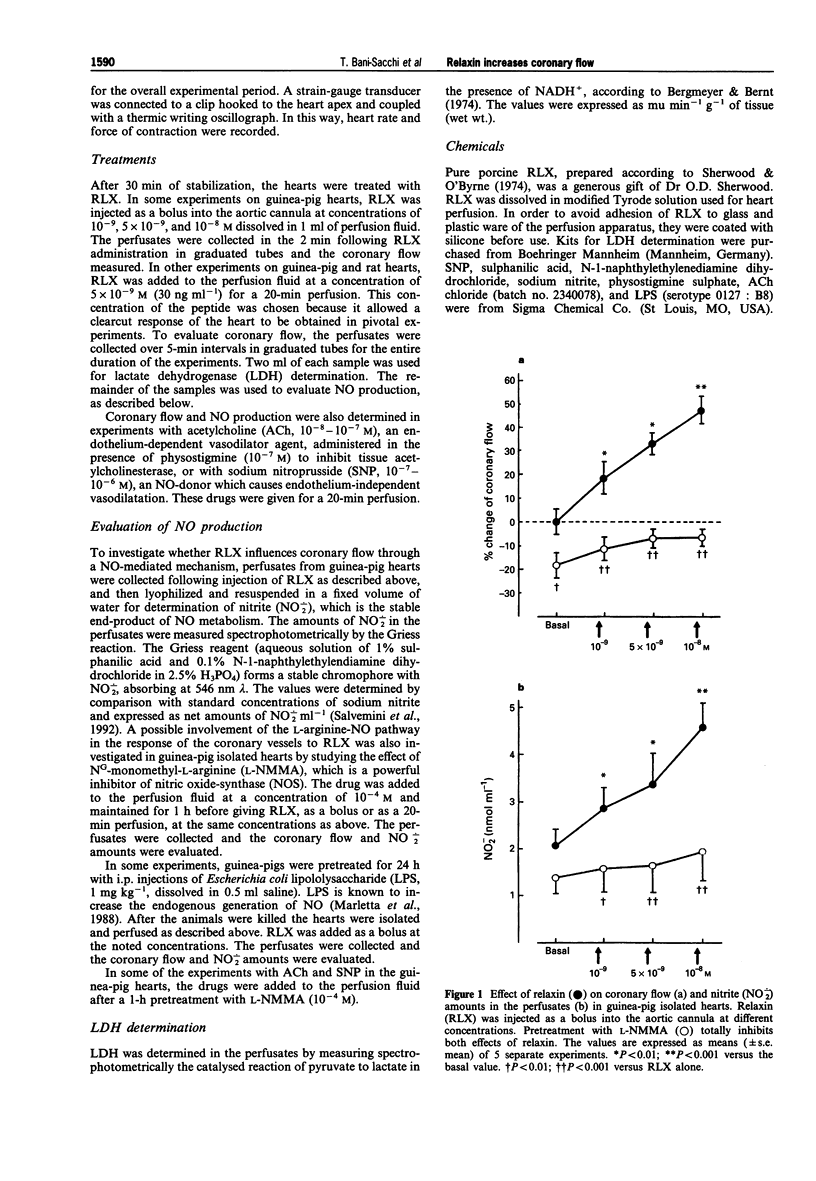
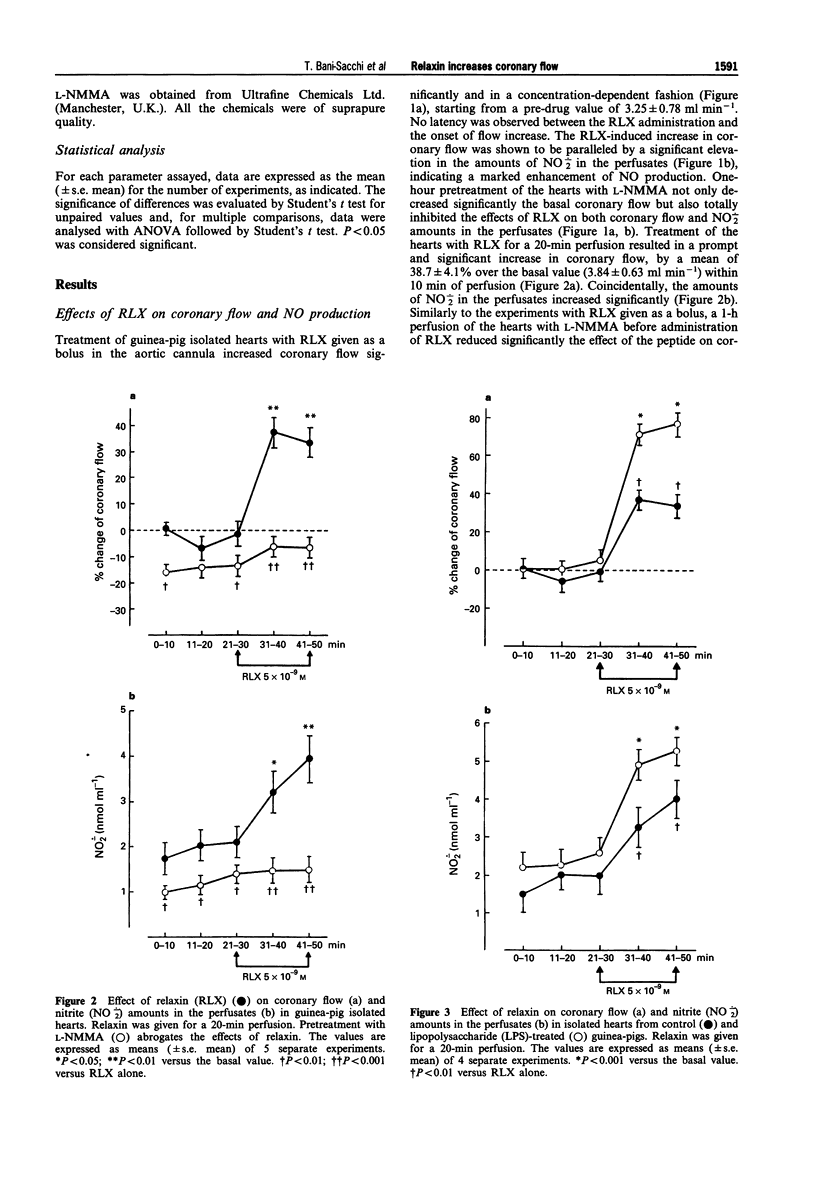
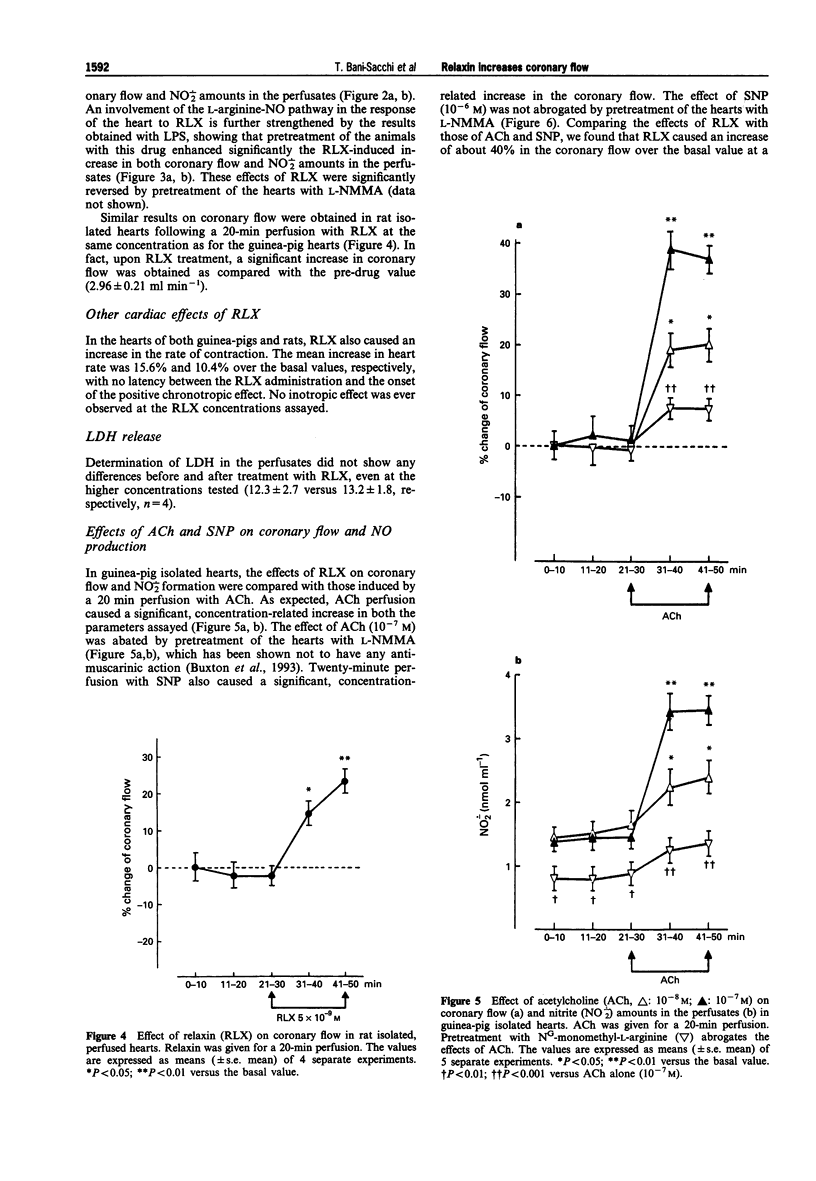
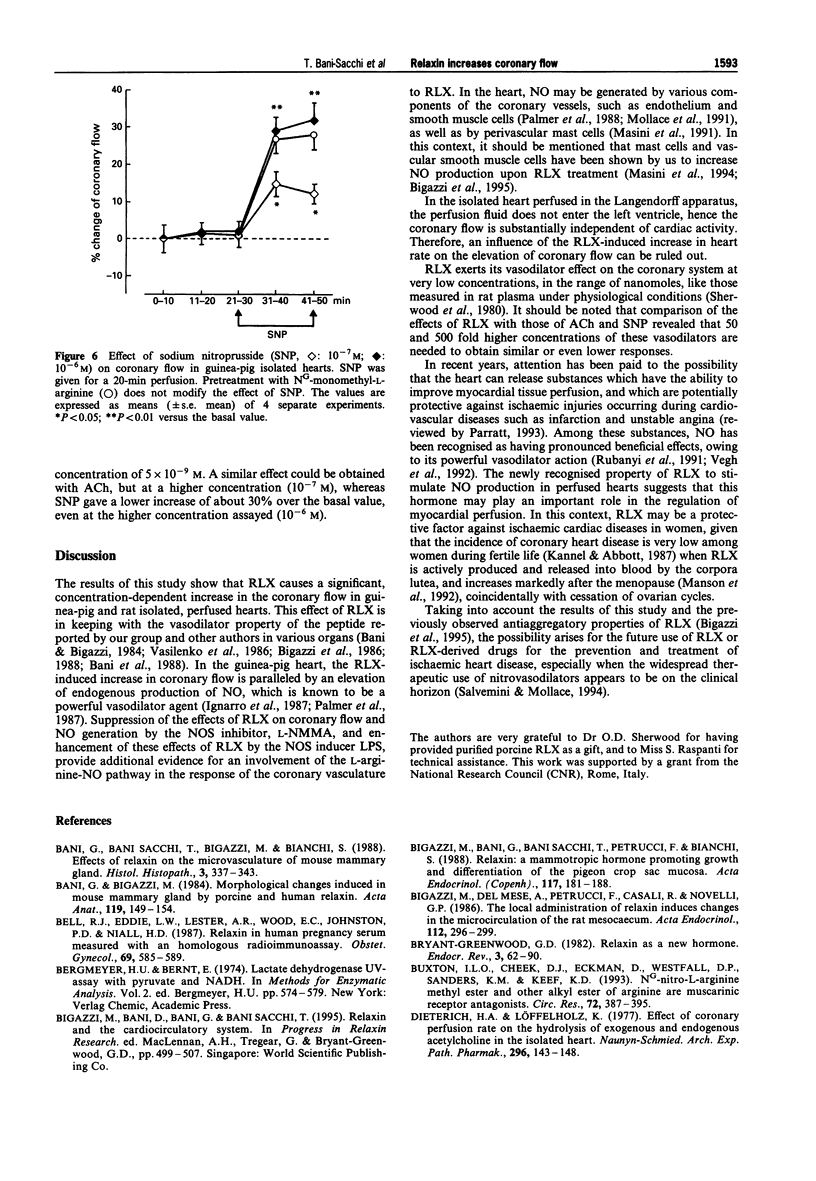
1. Relaxin (RLX) is a multifunctional hormone which, besides its role in pregnancy and parturition, has also been shown to influence the cardiovascular system. In this study, we investigated the effect of RLX on coronary flow of rat and guinea-pig hearts, isolated and perfused in a Langendorff apparatus. RLX was either added to the perfusion fluid at a concentration of 5 x 10(-9) M for a 20-min perfusion, or given as a bolus into the aortic cannula at concentrations of 10(-9) M, 5 x 10(-8) M dissolved in 1 ml of perfusion fluid. 2. RLX, given either for a 20-min perfusion or as a bolus in the aortic cannula to guinea-pig and rat isolated hearts, increased the coronary flow and the amount of nitrite, a stable end-product of nitric oxide (NO) metabolism, that appeared in the perfusates in a concentration-dependent fashion. 3. The increase in coronary flow and in nitrite in the perfusates induced by RLX was significantly reduced by pretreatment with the nitric oxide synthase (NOS) inhibitor, NG-monomethyl-L-arginine (L-NMMA, 10(-4) M). 4. The effects of RLX on coronary flow and nitrite amounts in the perfusates were compared with those induced by the endothelium-dependent vasodilator agent, acetylcholine (ACh, 10(-8)-10(-7) M), and by the endothelium-independent vasodilator agent, sodium nitroprusside (SNP, 10(-7)-10(-6) M). The results obtained show that RLX is more effective than ACh and SNP in increasing coronary flow.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bani G., Bani Sacchi T., Bigazzi M., Bianchi S. Effects of relaxin on the microvasculature of mouse mammary gland. Histol Histopathol. 1988 Oct;3(4):337–343. [PubMed] [Google Scholar]
- Bani G., Bigazzi M. Morphological changes induced in mouse mammary gland by porcine and human relaxin. Acta Anat (Basel) 1984;119(3):149–154. doi: 10.1159/000145877. [DOI] [PubMed] [Google Scholar]
- Bell R. J., Eddie L. W., Lester A. R., Wood E. C., Johnston P. D., Niall H. D. Relaxin in human pregnancy serum measured with an homologous radioimmunoassay. Obstet Gynecol. 1987 Apr;69(4):585–589. [PubMed] [Google Scholar]
- Bigazzi M., Bani G., Sacchi T. B., Petrucci F., Bianchi S. Relaxin: a mammotropic hormone promoting growth and differentiation of the pigeon crop sac mucosa. Acta Endocrinol (Copenh) 1988 Feb;117(2):181–188. doi: 10.1530/acta.0.1170181. [DOI] [PubMed] [Google Scholar]
- Bigazzi M., Del Mese A., Petrucci F., Casali R., Novelli G. P. The local administration of relaxin induces changes in the microcirculation of the rat mesocaecum. Acta Endocrinol (Copenh) 1986 Jun;112(2):296–299. doi: 10.1530/acta.0.1120296. [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood G. D. Relaxin as a new hormone. Endocr Rev. 1982 Winter;3(1):62–90. doi: 10.1210/edrv-3-1-62. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Cheek D. J., Eckman D., Westfall D. P., Sanders K. M., Keef K. D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993 Feb;72(2):387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Dieterich H. A., Löffelholz K. Effect of coronary perfusion rate on the hydrolysis of exogenous and endogenous acetylcholine in the isolated heart. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jan;296(2):143–148. doi: 10.1007/BF00508466. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakouris H., Eddie L. W., Summers R. J. Cardiac effects of relaxin in rats. Lancet. 1992 May 2;339(8801):1076–1078. doi: 10.1016/0140-6736(92)90665-p. [DOI] [PubMed] [Google Scholar]
- Manson J. E., Tosteson H., Ridker P. M., Satterfield S., Hebert P., O'Connor G. T., Buring J. E., Hennekens C. H. The primary prevention of myocardial infarction. N Engl J Med. 1992 May 21;326(21):1406–1416. doi: 10.1056/NEJM199205213262107. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Masini E., Bani D., Bigazzi M., Mannaioni P. F., Bani-Sacchi T. Effects of relaxin on mast cells. In vitro and in vivo studies in rats and guinea pigs. J Clin Invest. 1994 Nov;94(5):1974–1980. doi: 10.1172/JCI117549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini E., Salvemini D., Pistelli A., Mannaioni P. F., Vane J. R. Rat mast cells synthesize a nitric oxide like-factor which modulates the release of histamine. Agents Actions. 1991 May;33(1-2):61–63. doi: 10.1007/BF01993127. [DOI] [PubMed] [Google Scholar]
- Massicotte G., Parent A., St-Louis J. Blunted responses to vasoconstrictors in mesenteric vasculature but not in portal vein of spontaneously hypertensive rats treated with relaxin. Proc Soc Exp Biol Med. 1989 Mar;190(3):254–259. doi: 10.3181/00379727-190-42857. [DOI] [PubMed] [Google Scholar]
- Mollace V., Salvemini D., Anggard E., Vane J. Nitric oxide from vascular smooth muscle cells: regulation of platelet reactivity and smooth muscle cell guanylate cyclase. Br J Pharmacol. 1991 Nov;104(3):633–638. doi: 10.1111/j.1476-5381.1991.tb12481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff P. L., Cronin M. J., Lofgren J. A. Relaxin binding in the rat heart atrium. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2384–2388. doi: 10.1073/pnas.89.6.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff P. L., Ho W. H. Expression of relaxin mRNA and relaxin receptors in postnatal and adult rat brains and hearts. Localization and developmental patterns. J Biol Chem. 1993 Jul 15;268(20):15193–15199. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Parratt J. Endogenous myocardial protective (antiarrhythmic) substances. Cardiovasc Res. 1993 May;27(5):693–702. doi: 10.1093/cvr/27.5.693. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Ho E. H., Cantor E. H., Lumma W. C., Botelho L. H. Cytoprotective function of nitric oxide: inactivation of superoxide radicals produced by human leukocytes. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1392–1397. doi: 10.1016/0006-291x(91)92093-y. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Mollace V., Pistelli A., Anggard E., Vane J. Metabolism of glyceryl trinitrate to nitric oxide by endothelial cells and smooth muscle cells and its induction by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):982–986. doi: 10.1073/pnas.89.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood C. D., O'Byrne E. M. Purification and characterization of porcine relaxin. Arch Biochem Biophys. 1974 Jan;160(1):185–196. doi: 10.1016/s0003-9861(74)80025-1. [DOI] [PubMed] [Google Scholar]
- Sherwood O. D., Crnekovic V. E., Gordon W. L., Rutherford J. E. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980 Sep;107(3):691–698. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- Smit M. J., Bloemers S. M., Leurs R., Tertoolen L. G., Bast A., de Laat S. W., Timmerman H. Short-term desensitization of the histamine H1 receptor in human HeLa cells: involvement of protein kinase C dependent and independent pathways. Br J Pharmacol. 1992 Oct;107(2):448–455. doi: 10.1111/j.1476-5381.1992.tb12766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Louis J., Massicotte G. Chronic decrease of blood pressure by rat relaxin in spontaneously hypertensive rats. Life Sci. 1985 Oct 7;37(14):1351–1357. doi: 10.1016/0024-3205(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Vasilenko P., Mead J. P., Weidmann J. E. Uterine growth-promoting effects of relaxin: a morphometric and histological analysis. Biol Reprod. 1986 Nov;35(4):987–995. doi: 10.1095/biolreprod35.4.987. [DOI] [PubMed] [Google Scholar]
- Ward D. G., Thomas G. R., Cronin M. J. Relaxin increases rat heart rate by a direct action on the cardiac atrium. Biochem Biophys Res Commun. 1992 Jul 31;186(2):999–1005. doi: 10.1016/0006-291x(92)90845-c. [DOI] [PubMed] [Google Scholar]


