Abstract
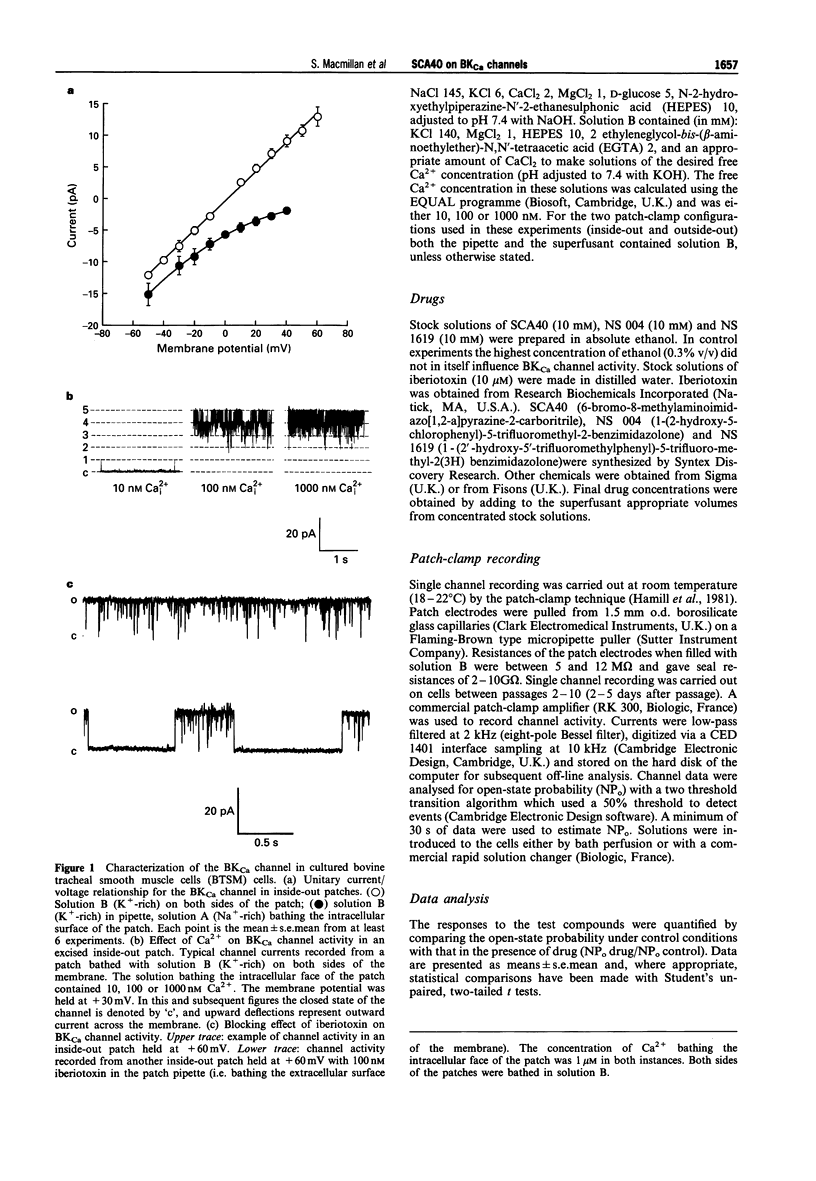
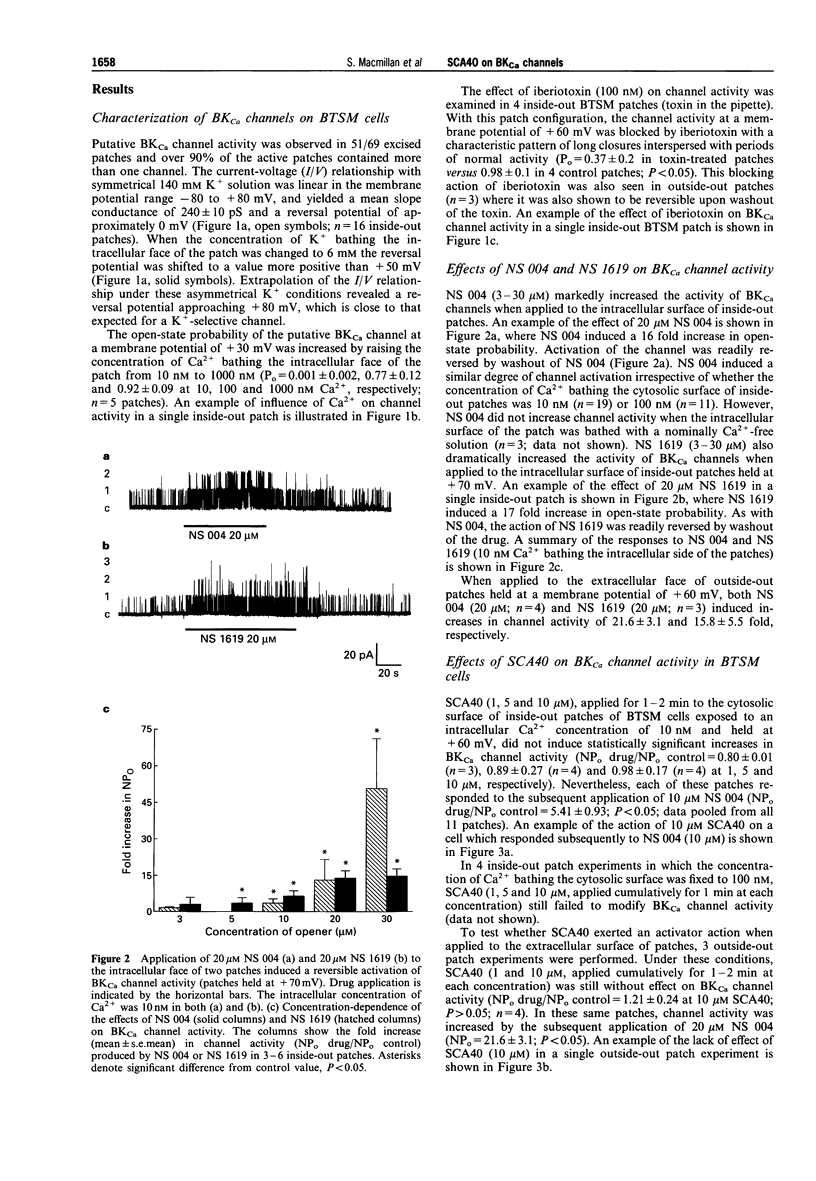
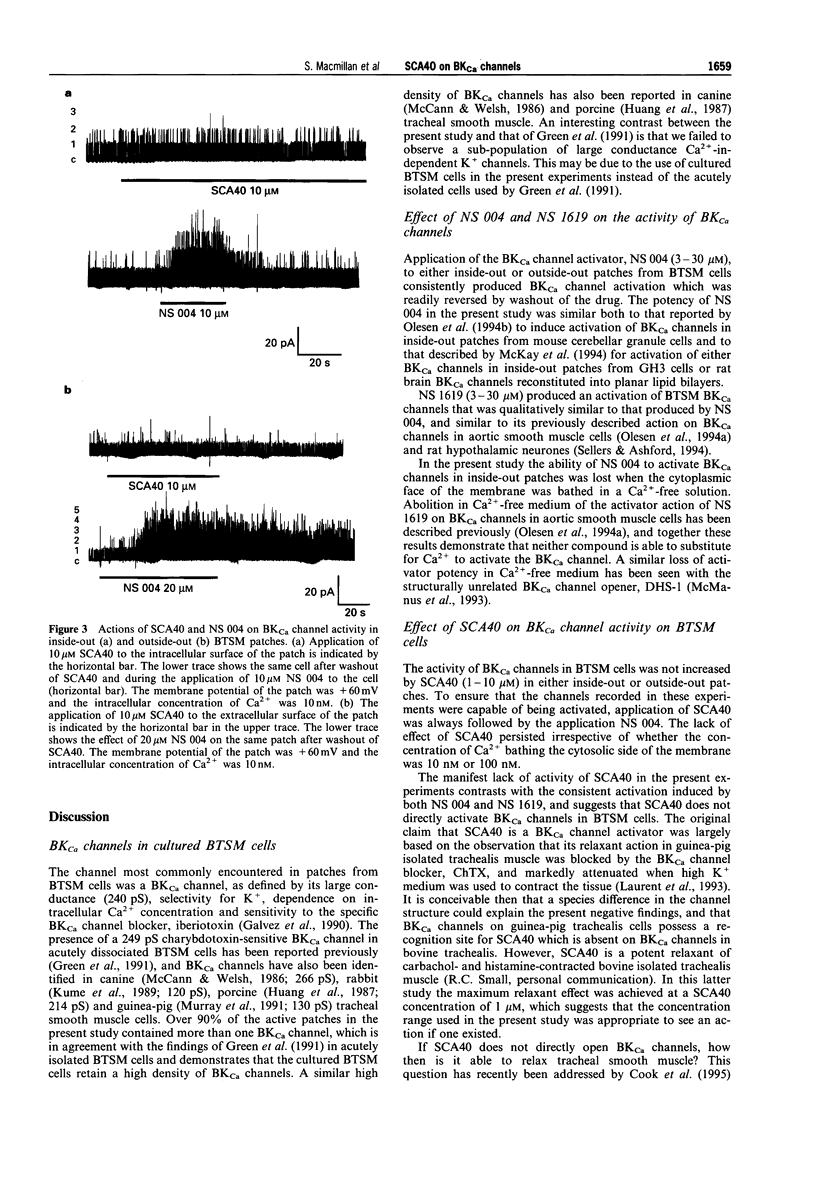
1. The effects of imidazopyrazine derivative, SCA40, on the activity of single large conductance, Ca(2+)-activated K+ (BKCa) channels in inside-out and outside-out patches from bovine tracheal smooth muscle (BTSM) cells in culture have been compared with those of two established BKCa channel openers, NS 004 and NS 1619. 2. The presence of BKCa channels on inside-out patches of BTSM membranes was confirmed by the single channel conductance (240 pS), selectivity for K+, dependence of channel activity on [Ca2+]i, and sensitivity to the selective BKCa channel blocker, iberiotoxin. 3. NS 004 and ND 1619 (3-30 microM) induced concentration-related increases in open state probability of BKCa channels when applied to either inside-out or outside-out BTSM patches, thus confirming that these compounds are activators of the BKCa channel in this preparation. 4. SCA40 (0.1-10 microM) had no effect on the activity of BKCa channels when applied to either inside-out or outside-out patches which subsequently responded to the application of NS 004 (10-20 microM). 5. It is concluded that SCA40 does not have a direct effect on BKCa channel activity in BTSM patches and that the previously reported relaxant action of SCA40 on tracheal smooth muscle is unlikely to be mediated by this mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnet P. A., Michel A., Laurent F., Sablayrolles C., Rechencq E., Mani J. C., Boucard M., Chapat J. P. Synthesis and antibronchospastic activity of 8-alkoxy- and 8-(alkylamino)imidazo[1,2-a]pyrazines. J Med Chem. 1992 Sep 4;35(18):3353–3358. doi: 10.1021/jm00096a008. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Archer K., Martin A., Buchheit K. H., Fozard J. R., Müller T., Miller A. J., Elliott K. R., Foster R. W., Small R. C. Further analysis of the mechanisms underlying the tracheal relaxant action of SCA40. Br J Pharmacol. 1995 Jan;114(1):143–151. doi: 10.1111/j.1476-5381.1995.tb14918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Green K. A., Foster R. W., Small R. C. A patch-clamp study of K(+)-channel activity in bovine isolated tracheal smooth muscle cells. Br J Pharmacol. 1991 Apr;102(4):871–878. doi: 10.1111/j.1476-5381.1991.tb12269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Laurent F., Michel A., Bonnet P. A., Chapat J. P., Boucard M. Evaluation of the relaxant effects of SCA40, a novel charybdotoxin-sensitive potassium channel opener, in guinea-pig isolated trachealis. Br J Pharmacol. 1993 Mar;108(3):622–626. doi: 10.1111/j.1476-5381.1993.tb12851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman S. D., Hamilton T. C. Potassium channel activator drugs: mechanism of action, pharmacological properties, and therapeutic potential. Med Res Rev. 1992 Mar;12(2):73–148. doi: 10.1002/med.2610120202. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay M. C., Dworetzky S. I., Meanwell N. A., Olesen S. P., Reinhart P. H., Levitan I. B., Adelman J. P., Gribkoff V. K. Opening of large-conductance calcium-activated potassium channels by the substituted benzimidazolone NS004. J Neurophysiol. 1994 May;71(5):1873–1882. doi: 10.1152/jn.1994.71.5.1873. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Harris G. H., Giangiacomo K. M., Feigenbaum P., Reuben J. P., Addy M. E., Burka J. F., Kaczorowski G. J., Garcia M. L. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993 Jun 22;32(24):6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Berry J. L., Cook S. J., Foster R. W., Green K. A., Small R. C. Guinea-pig isolated trachealis: the effects of charybdotoxin on mechanical activity, membrane potential changes and the activity of plasmalemmal K(+)-channels. Br J Pharmacol. 1991 Jul;103(3):1814–1818. doi: 10.1111/j.1476-5381.1991.tb09868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S. P., Munch E., Moldt P., Drejer J. Selective activation of Ca(2+)-dependent K+ channels by novel benzimidazolone. Eur J Pharmacol. 1994 Jan 4;251(1):53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Munch E., Wätjen F., Drejer J. NS 004--an activator of Ca(2+)-dependent K+ channels in cerebellar granule cells. Neuroreport. 1994 Apr 14;5(8):1001–1004. doi: 10.1097/00001756-199404000-00037. [DOI] [PubMed] [Google Scholar]
- Sellers A. J., Ashford M. L. Activation of BKCa channels in acutely dissociated neurones from the rat ventromedial hypothalamus by NS 1619. Br J Pharmacol. 1994 Nov;113(3):659–661. doi: 10.1111/j.1476-5381.1994.tb17041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twort C., Van Breemen C. Human airway smooth muscle in culture. Tissue Cell. 1988;20(3):339–344. doi: 10.1016/0040-8166(88)90069-9. [DOI] [PubMed] [Google Scholar]


