Abstract
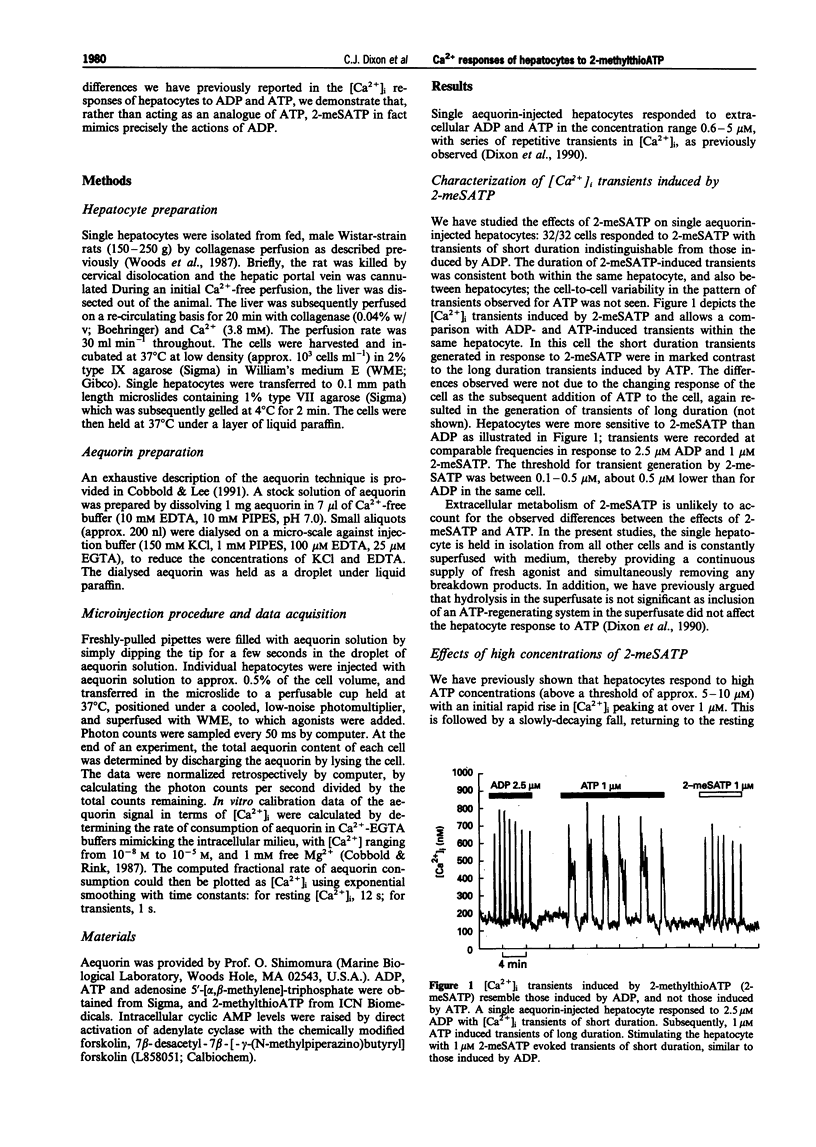
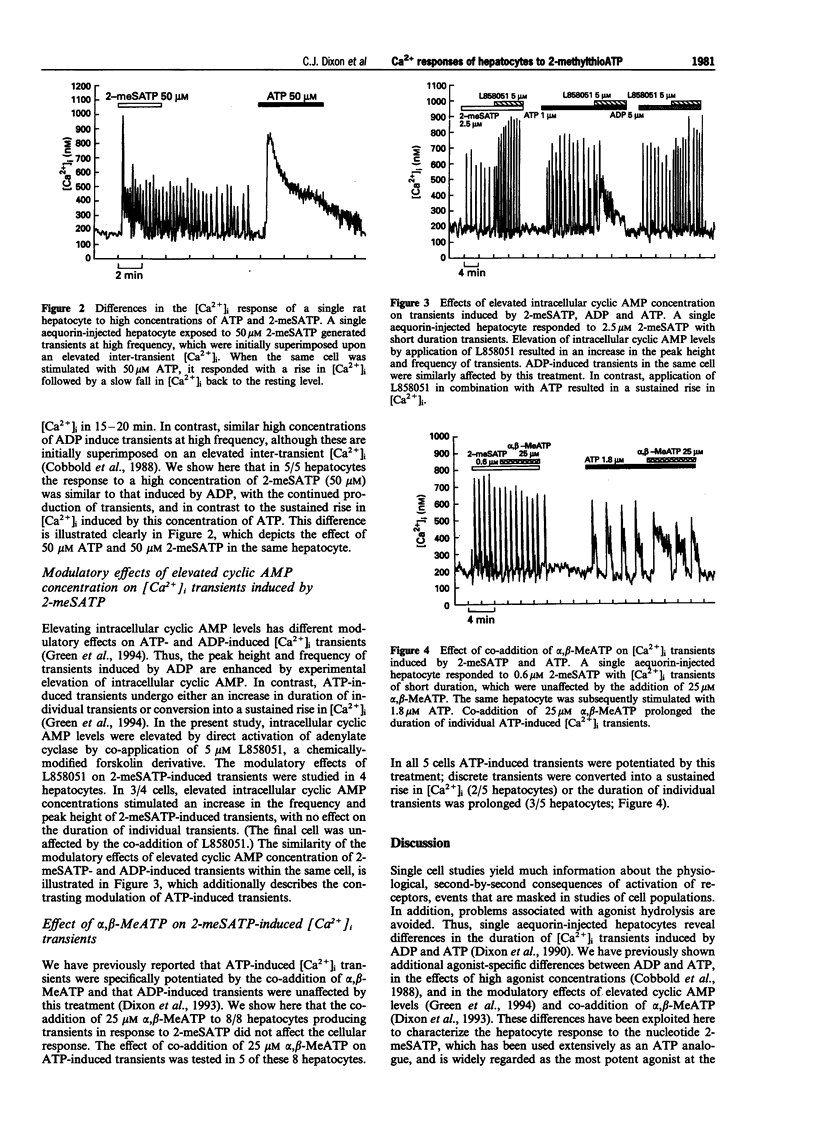
1. Aequorin-injected, single rat hepatocytes generate series of repetitive transients in cytosolic free calcium concentration ([Ca2+]i) when stimulated with agonists acting through the phosphoinositide signalling pathway, including ADP and ATP. We have previously described differences in the [Ca2+]i responses of aequorin-injected hepatocytes to ADP and ATP. 2. The effects of the phosphorothioate analogue of ATP, 2-methylthioATP (2-meSATP), have been examined on single rat hepatocytes. This analogue is belived to be the most potent agonist at the P2Y1 subclass of purinoceptor. 3. The [Ca2+]i transients induced by 2-meSATP were indistinguishable from those induced by ADP, and in contrast to those induced by ATP. 4. At hig concentrations, 2-meSATP and ADP both induced transients at high frequency. In contrast, hepatocytes responded to high concentrations of ATP with an initial rapid rise in [Ca2+]i, followed by a slowly decaying fall. 5. The modulatory effects of elevated intracellular cyclic AMP concentration were the same on both 2-meSATP- and ADP-induced [Ca2+]i transients; the peak height and frequency of transients were enhanced. ATP-induced transients, however, underwent either an increase in duration or conversion into a sustained rise in [Ca2+]i. 6. ATP-induced transients were specifically potentiated by the co-addition of alpha, beta-methyleneATP, whereas 2-meSATP- and ADP-induced transients were unaffected by this treatment. 7. We conclude that 2-meSATP acts at the same receptor as ADP on rat hepatocytes, and that this is distinct from teh receptor(s) mediating the effects of ATP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbracchio M. P., Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Bailey S. J., Hourani S. M. A study of the purinoceptors mediating contraction in the rat colon. Br J Pharmacol. 1990 Aug;100(4):753–756. doi: 10.1111/j.1476-5381.1990.tb14087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Brown H. A., Lazarowski E. R., Boucher R. C., Harden T. K. Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5'-nucleotide receptor in human airway epithelial cells. Mol Pharmacol. 1991 Nov;40(5):648–655. [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Cusack N. J., Pearson J. D. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988 Dec;95(4):1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P., Woods N., Wainwright J., Cuthbertson R. Single cell measurements in research on calcium-mobilising purinoceptors. J Recept Res. 1988;8(1-4):481–491. doi: 10.3109/10799898809049006. [DOI] [PubMed] [Google Scholar]
- Dixon C. J., Cobbold P. H., Green A. K. Adenosine 5'-[alpha beta-methylene]triphosphate potentiates the oscillatory cytosolic Ca2+ responses of hepatocytes to ATP, but not to ADP. Biochem J. 1993 Aug 1;293(Pt 3):757–760. doi: 10.1042/bj2930757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C. J., Woods N. M., Cuthbertson K. S., Cobbold P. H. Evidence for two Ca2(+)-mobilizing purinoceptors on rat hepatocytes. Biochem J. 1990 Jul 15;269(2):499–502. doi: 10.1042/bj2690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Gibb C. A., Singh S., Cook D. I., Poronnik P., Conigrave A. D. A nucleotide receptor that mobilizes Ca2+ in the mouse submandibular salivary cell line ST885. Br J Pharmacol. 1994 Apr;111(4):1135–1139. doi: 10.1111/j.1476-5381.1994.tb14863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. K., Cobbold P. H., Dixon C. J. Elevated intracellular cyclic AMP exerts different modulatory effects on cytosolic free Ca2+ oscillations induced by ADP and ATP in single rat hepatocytes. Biochem J. 1994 Sep 15;302(Pt 3):949–955. doi: 10.1042/bj3020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale P. A., Hill S. J. Increases in intracellular calcium via activation of an endogenous P2-purinoceptor in cultured CHO-K1 cells. Br J Pharmacol. 1993 Dec;110(4):1305–1310. doi: 10.1111/j.1476-5381.1993.tb13960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale P. A., Martin K. F., Alexander S. P., Hill S. J., Kendall D. A. Inositol 1,4,5-trisphosphate generation and calcium mobilisation via activation of an atypical P2 receptor in the neuronal cell line, N1E-115. Br J Pharmacol. 1992 Dec;107(4):1083–1087. doi: 10.1111/j.1476-5381.1992.tb13410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Characterization of the biological effects of 2-methylthio-ATP on rat hepatocytes: clear-cut differences with ATP. Br J Pharmacol. 1991 Oct;104(2):301–304. doi: 10.1111/j.1476-5381.1991.tb12426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Characterization of the liver P2-purinoceptor involved in the activation of glycogen phosphorylase. Biochem J. 1986 Dec 1;240(2):367–371. doi: 10.1042/bj2400367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S. The complex interaction of ATP and UTP with isolated hepatocytes. How many receptors? Gen Pharmacol. 1993 Mar;24(2):283–289. doi: 10.1016/0306-3623(93)90304-g. [DOI] [PubMed] [Google Scholar]
- Keppens S., Vandekerckhove A., De Wulf H. Characterization of the effects of adenosine 5'-[beta-thio]-diphosphate in rat liver. Br J Pharmacol. 1993 Mar;108(3):663–668. doi: 10.1111/j.1476-5381.1993.tb12858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandekerckhove A., De Wulf H. Extracellular ATP and UTP exert similar effects on rat isolated hepatocytes. Br J Pharmacol. 1992 Feb;105(2):475–479. doi: 10.1111/j.1476-5381.1992.tb14278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten M. D., Breittmayer J. P., Figarella C., Frelin C. ATP and UTP increase secretion of bronchial inhibitor by human tracheal gland cells in culture. Am J Physiol. 1993 Nov;265(5 Pt 1):L479–L484. doi: 10.1152/ajplung.1993.265.5.L479. [DOI] [PubMed] [Google Scholar]
- Milligan G. Mechanisms of multifunctional signalling by G protein-linked receptors. Trends Pharmacol Sci. 1993 Jun;14(6):239–244. doi: 10.1016/0165-6147(93)90019-g. [DOI] [PubMed] [Google Scholar]
- Murgo A. J., Contrera J. G., Sistare F. D. Evidence for separate calcium-signaling P2T and P2U purinoceptors in human megakaryocytic Dami cells. Blood. 1994 Mar 1;83(5):1258–1267. [PubMed] [Google Scholar]
- Murgo A. J., Sistare F. D. K562 leukemia cells express P2T (adenosine diphosphate) purinergic receptors. J Pharmacol Exp Ther. 1992 May;261(2):580–585. [PubMed] [Google Scholar]
- Murrin R. J., Boarder M. R. Neuronal "nucleotide" receptor linked to phospholipase C and phospholipase D? Stimulation of PC12 cells by ATP analogues and UTP. Mol Pharmacol. 1992 Mar;41(3):561–568. [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Palea S., Corsi M., Pietra C., Artibani W., Calpista A., Gaviraghi G., Trist D. G. ADP beta S induces contraction of the human isolated urinary bladder through a purinoceptor subtype different from P2X and P2Y. J Pharmacol Exp Ther. 1994 Apr;269(1):193–197. [PubMed] [Google Scholar]
- Raha S., de Souza L. R., Reed J. K. Intracellular signalling by nucleotide receptors in PC12 pheochromocytoma cells. J Cell Physiol. 1993 Mar;154(3):623–630. doi: 10.1002/jcp.1041540322. [DOI] [PubMed] [Google Scholar]
- Reimer W. J., Dixon S. J. Extracellular nucleotides elevate [Ca2+]i in rat osteoblastic cells by interaction with two receptor subtypes. Am J Physiol. 1992 Nov;263(5 Pt 1):C1040–C1048. doi: 10.1152/ajpcell.1992.263.5.C1040. [DOI] [PubMed] [Google Scholar]
- Robb S., Cheek T. R., Hannan F. L., Hall L. M., Midgley J. M., Evans P. D. Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. EMBO J. 1994 Mar 15;13(6):1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P., Feolde E., Breittmayer J. P., Frelin C. Characterization of the effects of 2-methylthio-ATP and 2-chloro-ATP on brain capillary endothelial cells: similarities to ADP and differences from ATP. Br J Pharmacol. 1994 Jul;112(3):775–780. doi: 10.1111/j.1476-5381.1994.tb13146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. F., McKechnie K., Dainty I. A., Boarder M. R. P2Y purinoceptor and nucleotide receptor-induced relaxation of precontracted bovine aortic collateral artery rings: differential sensitivity to suramin and indomethacin. J Pharmacol Exp Ther. 1994 Feb;268(2):881–887. [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


