Abstract
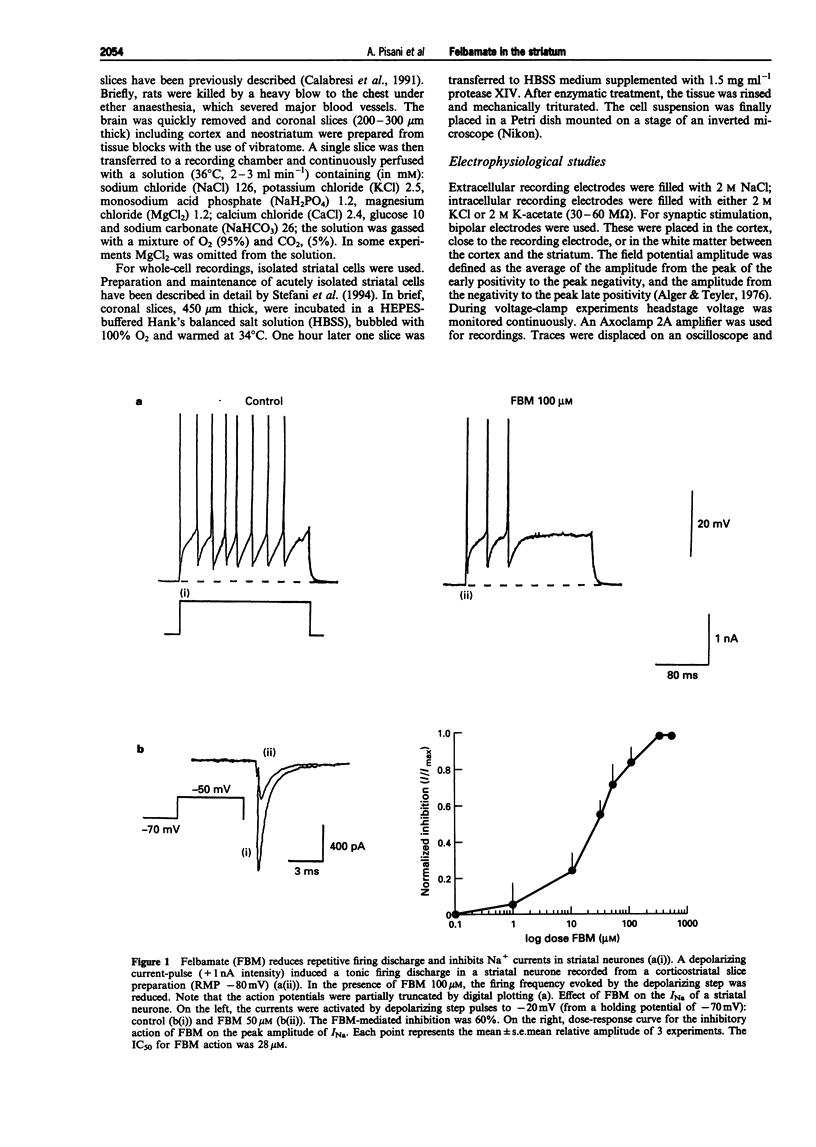
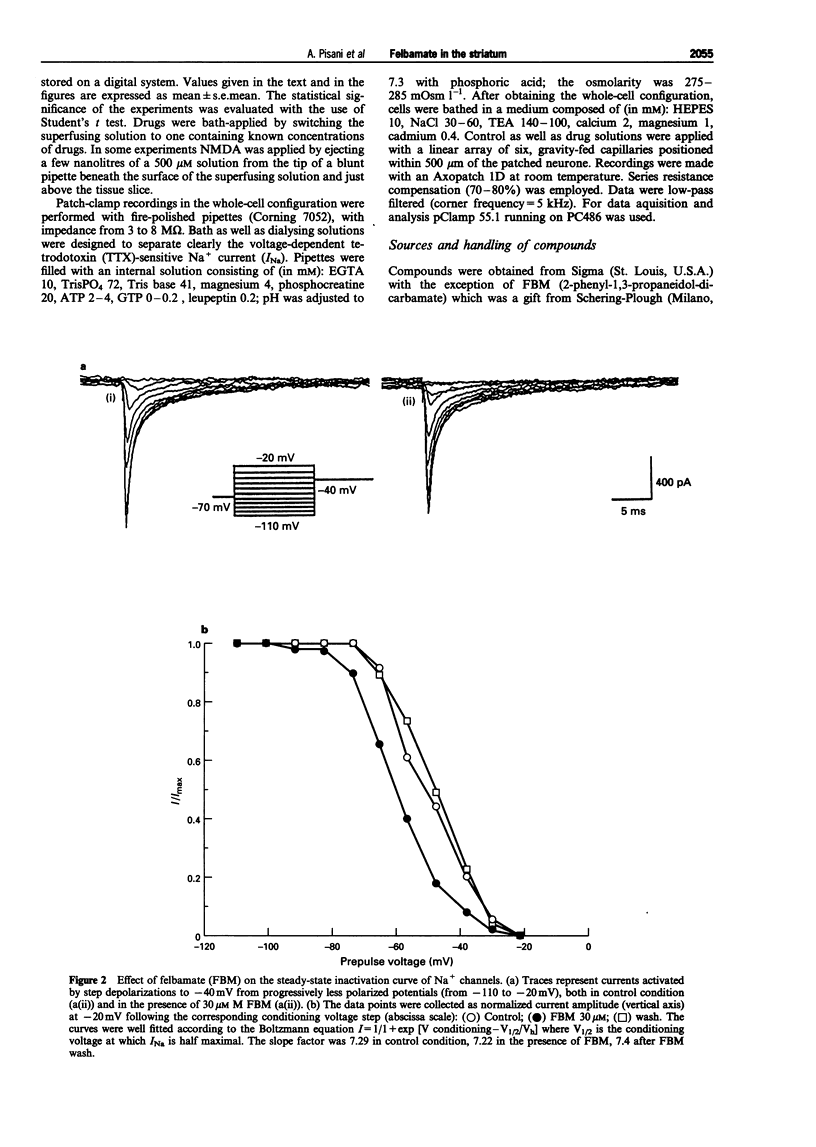
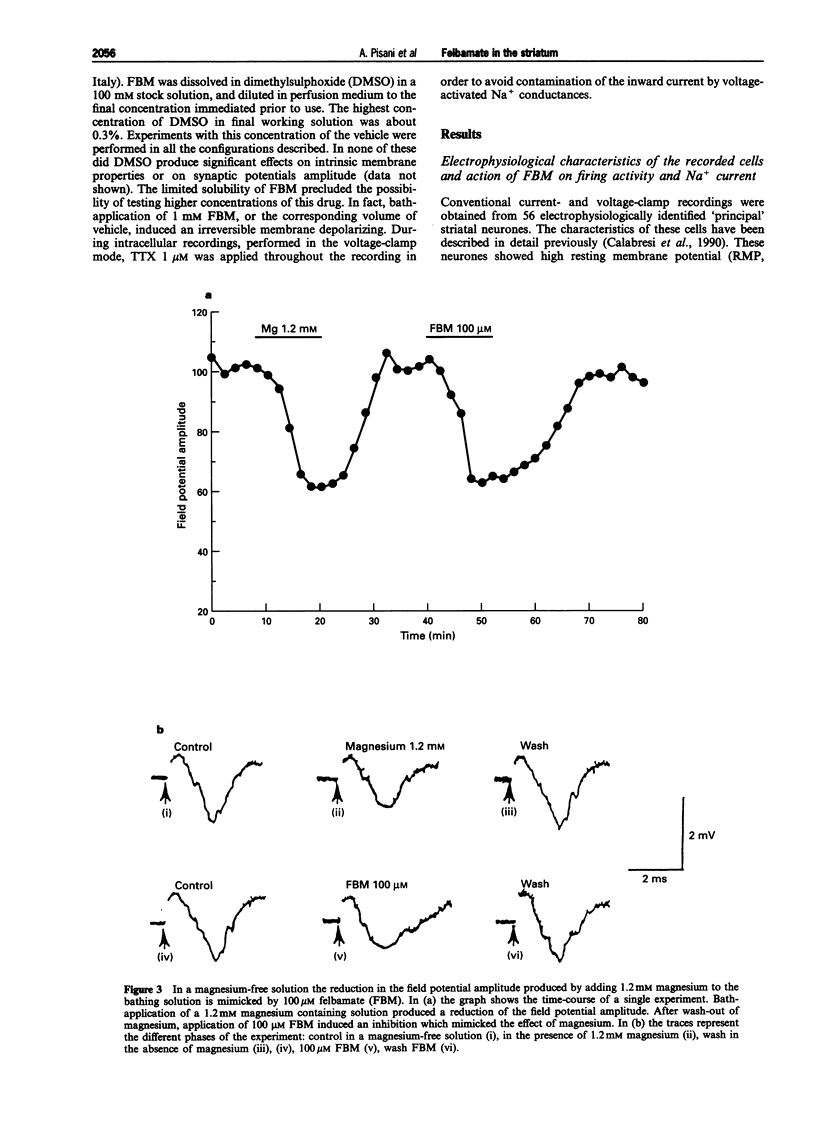
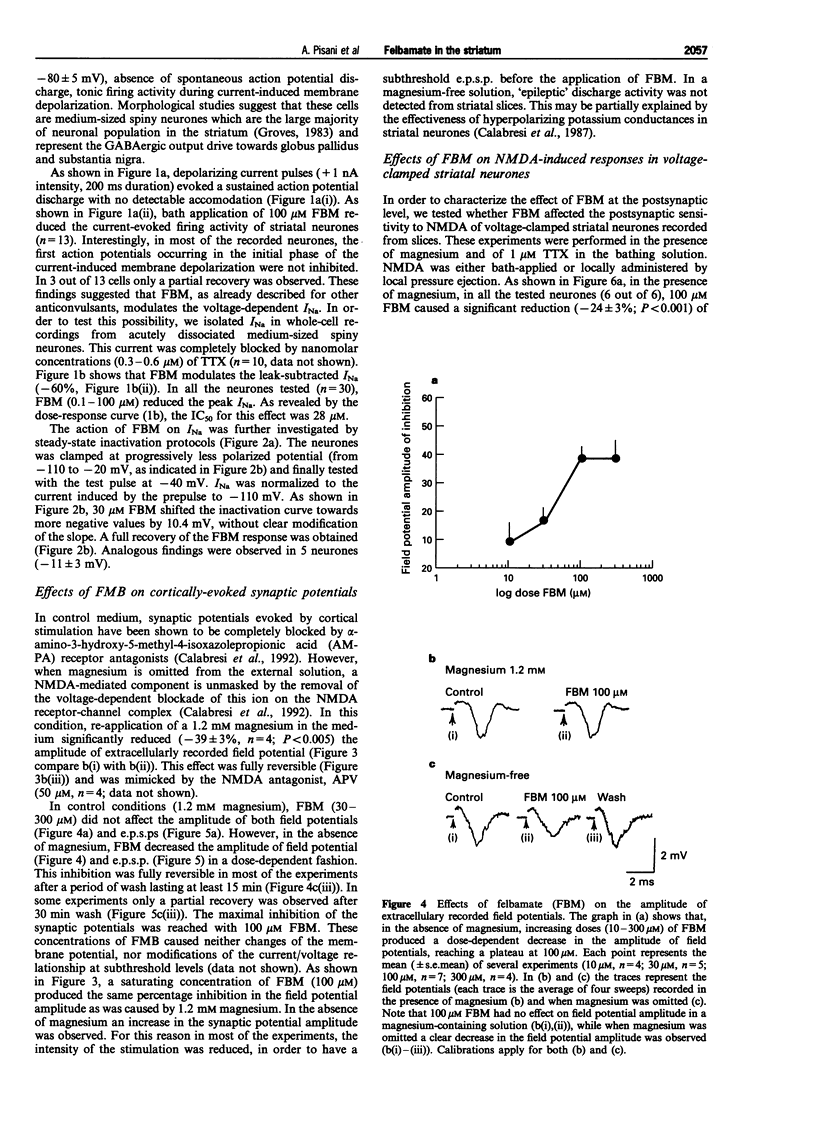
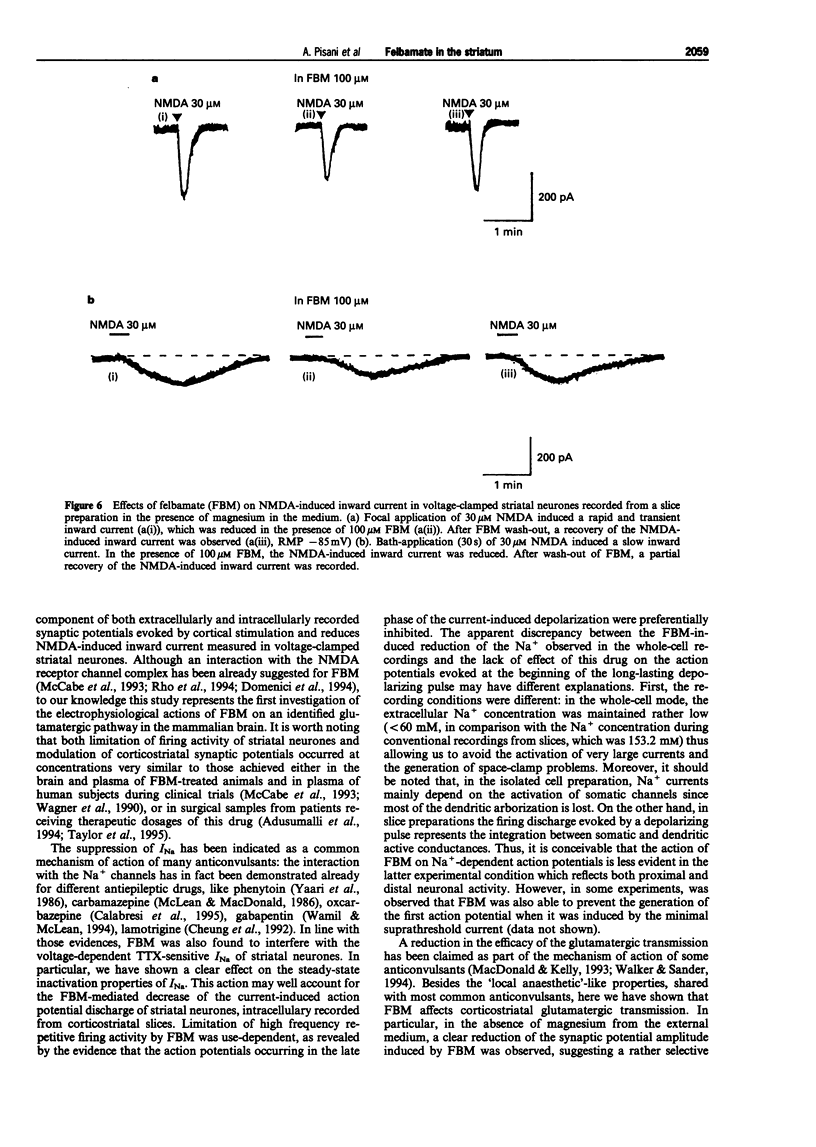
1. We have investigated the effects of the anticonvulsant drug, felbamate (FBM), on striatal neurones, recorded in vitro by using both intracellular and extracellular conventional recordings in slices and whole-cell recordings in acutely isolated neurones. 2. FBM, at therapeutically relevant concentrations (30-300 microM) showed multiple mechanisms of action. Like other antiepileptic drugs, FBM (30-300 microM) showed a direct inhibitory action on current-evoked firing discharge of striatal neurones. A patch-clamp analysis of this effect revealed a dose-related reduction of voltage-dependent sodium (Na+) currents (10-100 microM), with a half inhibiton dose (IC50) value of 28 microM. 3. We also tested whether FBM affected corticostriatal glutamate transmission. In control medium (1.2 mM external magnesium), both extracellularly recorded field potentials and intracellularly recorded excitatory postsynaptic potentials (e.p.s.ps) evoked by cortical stimulation were no affected by bath application of 30-300 microM FBM. 4. When magnesium was removed from the perfusing solution, a procedure which reveals a N-methyl-D-aspartate (NMDA)-mediated component in the corticostriatal synaptic potential, FBM (30-300 microM) produced a dose-dependent reduction of the amplitude of both the field potential and the e.p.s.p. 5. FBM reduced the inward currents produced either by bath or by focal applications of 30 microM NMDA, finding consistent with the hypothesis that the observed reduction of the NMDA-mediated component of the synaptic potentials may be caused at postsynaptic level. 6. The reduction of the NMDA-mediated component of the synaptic transmission by FBM and its depressant effect on the voltage-dependent Na+ channels, may account for the antiepileptic action of this drug. Moreover, the pharmacological properties of FBM might render this drug interesting as a neuroprotectant agent.
Full text
PDF
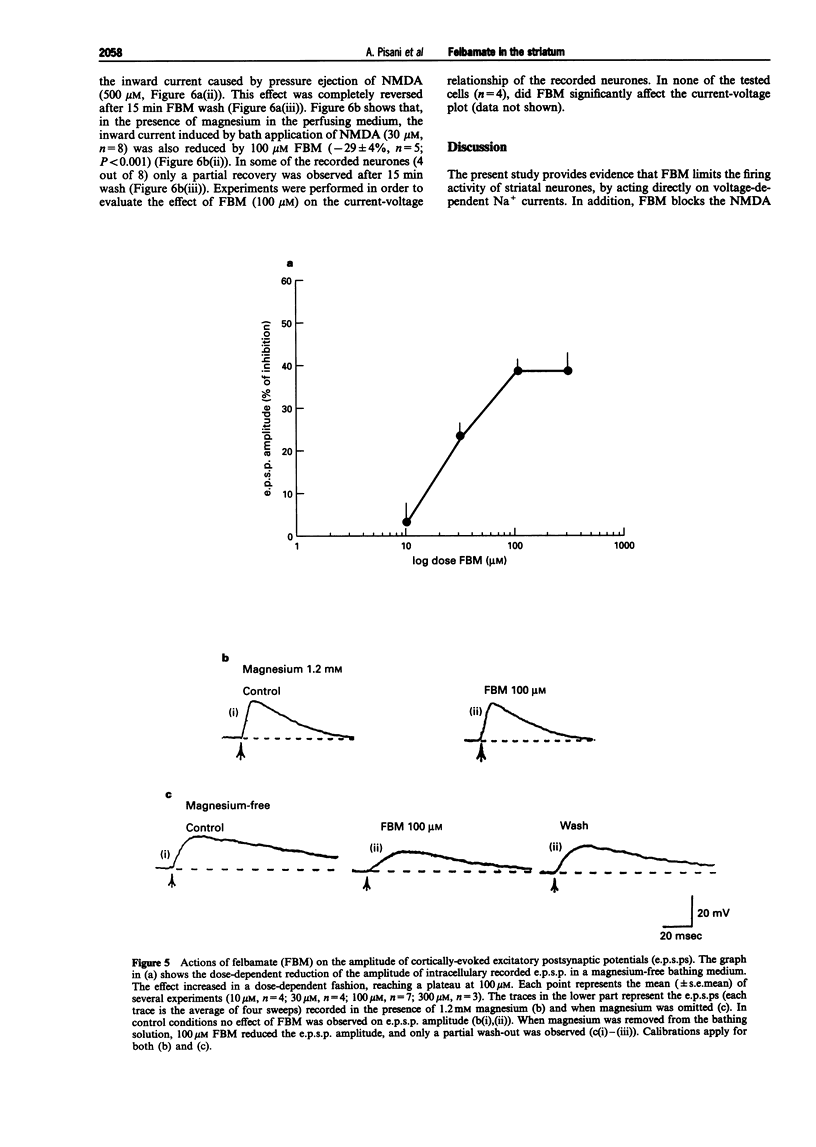


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adusumalli V. E., Wichmann J. K., Kucharczyk N., Kamin M., Sofia R. D., French J., Sperling M., Bourgeois B., Devinsky O., Dreifuss F. E. Drug concentrations in human brain tissue samples from epileptic patients treated with felbamate. Drug Metab Dispos. 1994 Jan-Feb;22(1):168–170. [PubMed] [Google Scholar]
- Alger B. E., Teyler T. J. Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976 Jul 16;110(3):463–480. doi: 10.1016/0006-8993(76)90858-1. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Brouillet E., Jenkins B., Henshaw R., Rosen B., Hyman B. T. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem. 1993 Sep;61(3):1147–1150. doi: 10.1111/j.1471-4159.1993.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Beal M. F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992 Feb;31(2):119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- Burdette D. E., Sackellares J. C. Felbamate pharmacology and use in epilepsy. Clin Neuropharmacol. 1994 Oct;17(5):389–402. doi: 10.1097/00002826-199410000-00001. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N. B., Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons. II. An in vitro analysis. J Neurophysiol. 1990 Apr;63(4):663–675. doi: 10.1152/jn.1990.63.4.663. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N. B., De Murtas M., Bernardi G. Involvement of GABA systems in feedback regulation of glutamate-and GABA-mediated synaptic potentials in rat neostriatum. J Physiol. 1991;440:581–599. doi: 10.1113/jphysiol.1991.sp018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Misgeld U., Dodt H. U. Intrinsic membrane properties of neostriatal neurons can account for their low level of spontaneous activity. Neuroscience. 1987 Jan;20(1):293–303. doi: 10.1016/0306-4522(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Pisani A., Mercuri N. B., Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur J Neurosci. 1992;4(10):929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Pisani A., Mercuri N. B., Bernardi G. Post-receptor mechanisms underlying striatal long-term depression. J Neurosci. 1994 Aug;14(8):4871–4881. doi: 10.1523/JNEUROSCI.14-08-04871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H., Kamp D., Harris E. An in vitro investigation of the action of lamotrigine on neuronal voltage-activated sodium channels. Epilepsy Res. 1992 Nov;13(2):107–112. doi: 10.1016/0920-1211(92)90065-2. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Koh J. Y., Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988 Jan;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulos A., Stafstrom C., Thurber S., Hyde P., Mikati M., Holmes G. L. Neuroprotective effect of felbamate after kainic acid-induced status epilepticus. Epilepsia. 1993 Mar-Apr;34(2):359–366. doi: 10.1111/j.1528-1157.1993.tb02422.x. [DOI] [PubMed] [Google Scholar]
- De Sarro G., Ongini E., Bertorelli R., Aguglia U., De Sarro A. Excitatory amino acid neurotransmission through both NMDA and non-NMDA receptors is involved in the anticonvulsant activity of felbamate in DBA/2 mice. Eur J Pharmacol. 1994 Sep 1;262(1-2):11–19. doi: 10.1016/0014-2999(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Domenici M. R., Sagratella S., Ongini E., Longo R., Scotti de Carolis A. Felbamate displays in vitro antiepileptic effects as a broad spectrum excitatory amino acid receptor antagonist. Eur J Pharmacol. 1994 Dec 27;271(2-3):259–263. doi: 10.1016/0014-2999(94)90782-x. [DOI] [PubMed] [Google Scholar]
- Greene J. G., Porter R. H., Eller R. V., Greenamyre J. T. Inhibition of succinate dehydrogenase by malonic acid produces an "excitotoxic" lesion in rat striatum. J Neurochem. 1993 Sep;61(3):1151–1154. doi: 10.1111/j.1471-4159.1993.tb03634.x. [DOI] [PubMed] [Google Scholar]
- Groves P. M. A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Res. 1983 Mar;286(2):109–132. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Kretschmer B. D. Felbamate, an anti-convulsive drug, has anti-parkinsonian potential in rats. Neurosci Lett. 1994 Sep 26;179(1-2):115–118. doi: 10.1016/0304-3940(94)90948-2. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Kelly K. M. Antiepileptic drug mechanisms of action. Epilepsia. 1993;34 (Suppl 5):S1–S8. doi: 10.1111/j.1528-1157.1993.tb05918.x. [DOI] [PubMed] [Google Scholar]
- McCabe R. T., Wasterlain C. G., Kucharczyk N., Sofia R. D., Vogel J. R. Evidence for anticonvulsant and neuroprotectant action of felbamate mediated by strychnine-insensitive glycine receptors. J Pharmacol Exp Ther. 1993 Mar;264(3):1248–1252. [PubMed] [Google Scholar]
- McLean M. J., Macdonald R. L. Carbamazepine and 10,11-epoxycarbamazepine produce use- and voltage-dependent limitation of rapidly firing action potentials of mouse central neurons in cell culture. J Pharmacol Exp Ther. 1986 Aug;238(2):727–738. [PubMed] [Google Scholar]
- Neafsey E. J., Chuman C. M., Ward A. A., Jr Propagation of focal cortical epileptiform discharge to the basal ganglia. Exp Neurol. 1979 Oct;66(1):97–108. doi: 10.1016/0014-4886(79)90066-9. [DOI] [PubMed] [Google Scholar]
- Nightingale S. L. From the Food and Drug Administration. JAMA. 1994 Oct 5;272(13):995–995. doi: 10.1001/jama.272.13.995. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Cuenod M. Glutamate release in vitro from corticostriatal terminals. Brain Res. 1979 Oct 26;176(1):185–188. doi: 10.1016/0006-8993(79)90884-9. [DOI] [PubMed] [Google Scholar]
- Rho J. M., Donevan S. D., Rogawski M. A. Mechanism of action of the anticonvulsant felbamate: opposing effects on N-methyl-D-aspartate and gamma-aminobutyric acidA receptors. Ann Neurol. 1994 Feb;35(2):229–234. doi: 10.1002/ana.410350216. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Stefani A., Pisani A., Mercuri N. B., Bernardi G., Calabresi P. Activation of metabotropic glutamate receptors inhibits calcium currents and GABA-mediated synaptic potentials in striatal neurons. J Neurosci. 1994 Nov;14(11 Pt 1):6734–6743. doi: 10.1523/JNEUROSCI.14-11-06734.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveinbjornsdottir S., Sander J. W., Upton D., Thompson P. J., Patsalos P. N., Hirt D., Emre M., Lowe D., Duncan J. S. The excitatory amino acid antagonist D-CPP-ene (SDZ EAA-494) in patients with epilepsy. Epilepsy Res. 1993 Oct;16(2):165–174. doi: 10.1016/0920-1211(93)90031-2. [DOI] [PubMed] [Google Scholar]
- Swinyard E. A., Sofia R. D., Kupferberg H. J. Comparative anticonvulsant activity and neurotoxicity of felbamate and four prototype antiepileptic drugs in mice and rats. Epilepsia. 1986 Jan-Feb;27(1):27–34. doi: 10.1111/j.1528-1157.1986.tb03497.x. [DOI] [PubMed] [Google Scholar]
- Taylor L. A., McQuade R. D., Tice M. A. Felbamate, a novel antiepileptic drug, reverses N-methyl-D-aspartate/glycine-stimulated increases in intracellular Ca2+ concentration. Eur J Pharmacol. 1995 Apr 28;289(2):229–233. doi: 10.1016/0922-4106(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Kamatchi G. L., Sofia R. D. Effect of anticonvulsant felbamate on GABAA receptor system. Epilepsia. 1991 May-Jun;32(3):389–391. doi: 10.1111/j.1528-1157.1991.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Turski L., Cavalheiro E. A., Turski W. A., Meldrum B. S. Excitatory neurotransmission within substantia nigra pars reticulata regulates threshold for seizures produced by pilocarpine in rats: effects of intranigral 2-amino-7-phosphonoheptanoate and N-methyl-D-aspartate. Neuroscience. 1986 May;18(1):61–77. doi: 10.1016/0306-4522(86)90179-x. [DOI] [PubMed] [Google Scholar]
- Turski L., Turski W. A. Towards an understanding of the role of glutamate in neurodegenerative disorders: energy metabolism and neuropathology. Experientia. 1993 Dec 15;49(12):1064–1072. doi: 10.1007/BF01929915. [DOI] [PubMed] [Google Scholar]
- Walker M. C., Sander J. W. Developments in antiepileptic drug therapy. Curr Opin Neurol. 1994 Apr;7(2):131–139. doi: 10.1097/00019052-199404000-00009. [DOI] [PubMed] [Google Scholar]
- Wallis R. A., Panizzon K. L., Fairchild M. D., Wasterlain C. G. Protective effects of felbamate against hypoxia in the rat hippocampal slice. Stroke. 1992 Apr;23(4):547–551. doi: 10.1161/01.str.23.4.547. [DOI] [PubMed] [Google Scholar]
- Wamil A. W., McLean M. J. Limitation by gabapentin of high frequency action potential firing by mouse central neurons in cell culture. Epilepsy Res. 1994 Jan;17(1):1–11. doi: 10.1016/0920-1211(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Wamsley J. K., Sofia R. D., Faull R. L., Narang N., Ary T., McCabe R. T. Interaction of felbamate with [3H]DCKA-labeled strychnine-insensitive glycine receptors in human postmortem brain. Exp Neurol. 1994 Oct;129(2):244–250. doi: 10.1006/exnr.1994.1166. [DOI] [PubMed] [Google Scholar]
- Wasterlain C. G., Adams L. M., Hattori H., Schwartz P. H. Felbamate reduces hypoxic-ischemic brain damage in vivo. Eur J Pharmacol. 1992 Mar 3;212(2-3):275–278. doi: 10.1016/0014-2999(92)90343-3. [DOI] [PubMed] [Google Scholar]
- White H. S., Wolf H. H., Swinyard E. A., Skeen G. A., Sofia R. D. A neuropharmacological evaluation of felbamate as a novel anticonvulsant. Epilepsia. 1992 May-Jun;33(3):564–572. doi: 10.1111/j.1528-1157.1992.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Yaari Y., Selzer M. E., Pincus J. H. Phenytoin: mechanisms of its anticonvulsant action. Ann Neurol. 1986 Aug;20(2):171–184. doi: 10.1002/ana.410200202. [DOI] [PubMed] [Google Scholar]


