Abstract
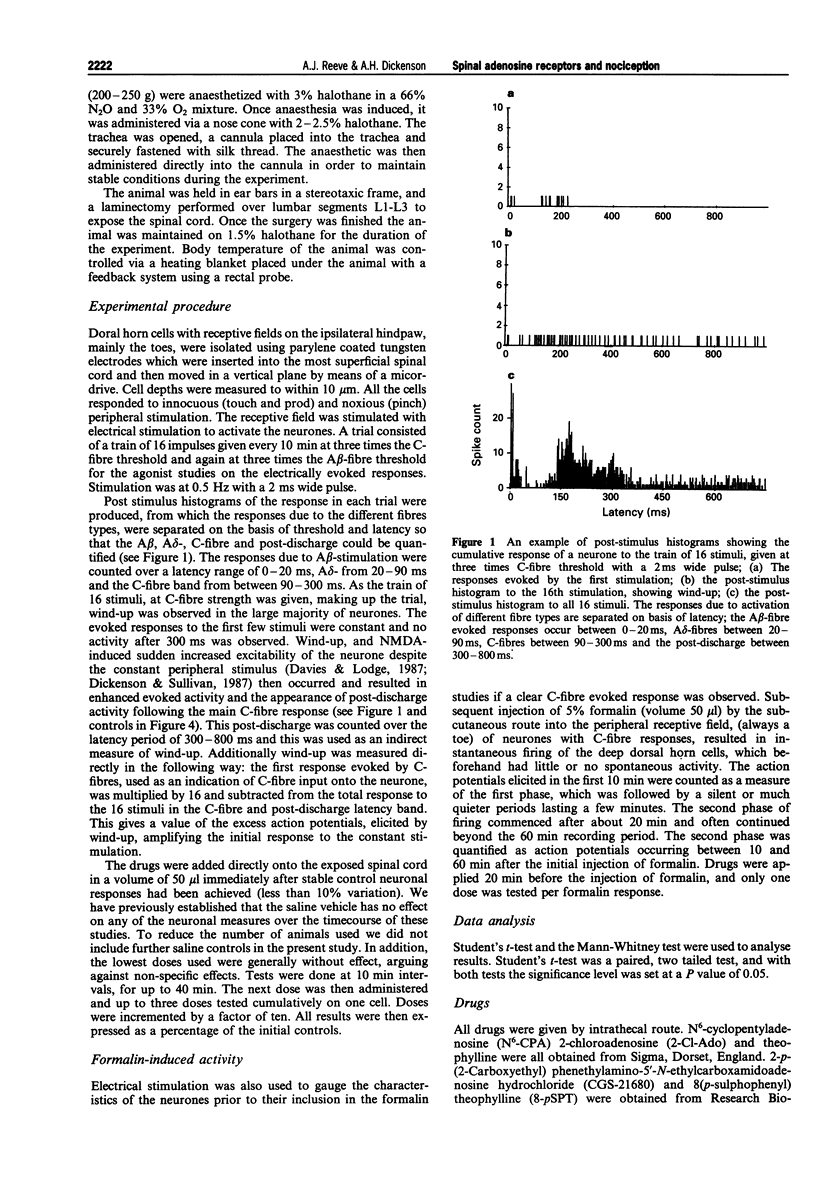
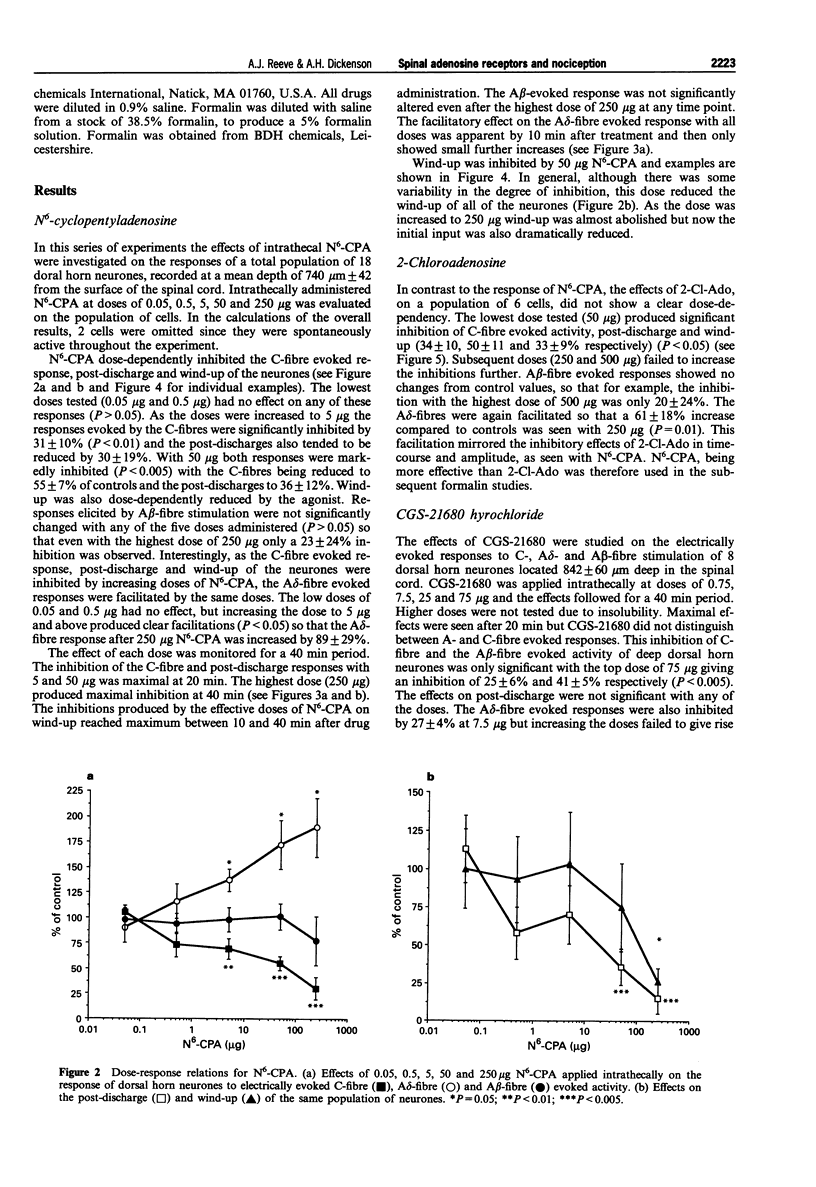
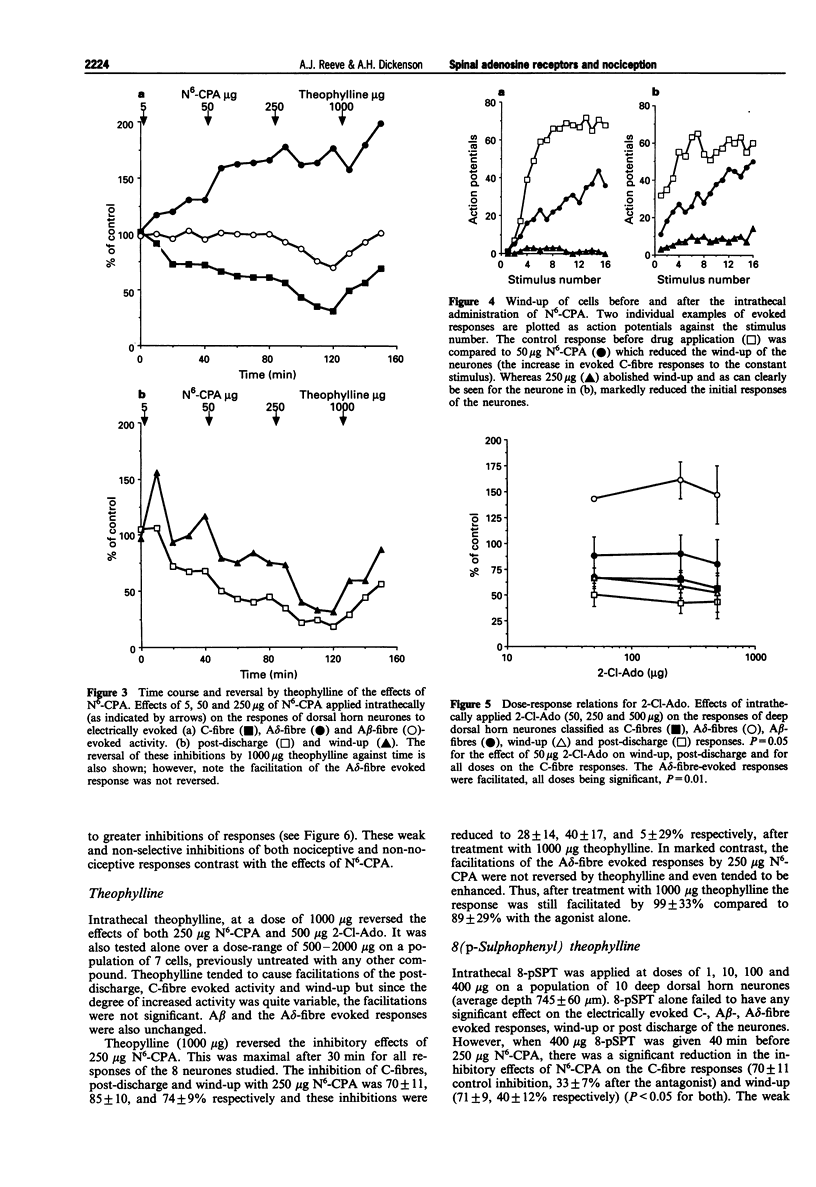
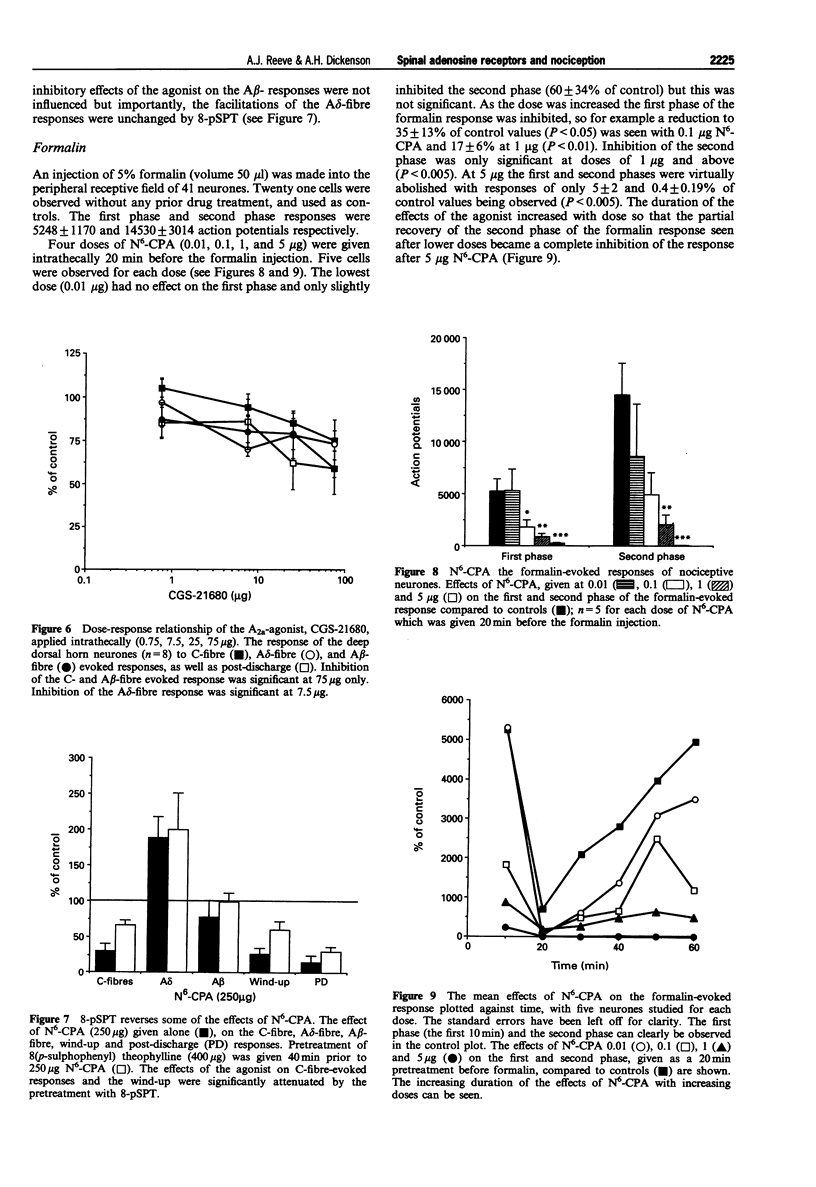
1. We describe here the effects of intrathecal selective adenosine receptor agonists on acute and more persistent evoked responses of dorsal horn nociceptive neurones recorded in intact rats anaesthetized with halothane. 2. The effects of the A1 receptor agonist, N6-cyclopentyladenosine and the non-selective agonist 2-chloroadenosine as well as the A2a receptor agonist, 2-p-(2-carboxyethyl) phenethylamino-5'-N-ethylcarboxamidoadenosine hydrochloride were gauged on the C-, A delta-, A beta-fibre, post-discharge and wind-up responses produced by peripheral tanscutaneous stimulation. The antagonists, theophylline and 8(p-sulphophenyl) theophylline were also tested alone and to reverse the agonist effects. 3. Subcutaneous formalin (5%) was used to produce a more prolonged nociceptive response initiated by peripheral inflammation. 4. Both N6-cyclopentyladenosine and 2-chloroadenosine produced inhibitions of the C-fibre evoked responses, wind-up and post-discharge of the neurones with no significant effects on the A beta responses. By contrast, the A delta evoked responses were facilitated over the same time course and dose-range as the inhibitions. N6-cyclopentyladenosine was more potent and effective than 2-chloroadenosine. In marked contrast to these agonists, the A2a agonist produced only weak non-specific inhibitions. Theophylline and 8(p-sulphophenyl) theophylline alone had no effect on the acute responses but prevented or reversed inhibitory effects of N6-cyclopentyladenosine. 5. The formalin response was markedly inhibited by spinal N6-cyclopentyladenosine with both the acute first phase and more prolonged second phase being dose-dependently inhibited. N6-cyclopentyladenosine was considerably more potent on the formalin response than on the other neuronal measures.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba H., Yoshimura M., Nishi S., Shimoji K. Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro. J Physiol. 1994 Jul 1;478(Pt 1):87–99. doi: 10.1113/jphysiol.1994.sp020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas K. M., Newby A. C., Wilson V. S., Snyder S. H. Adenosine-containing neurons in the brain localized by immunocytochemistry. J Neurosci. 1986 Jul;6(7):1952–1961. doi: 10.1523/JNEUROSCI.06-07-01952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman V., Dickenson A. H. The effect of intrathecal administration of RP67580, a potent neurokinin 1 antagonist on nociceptive transmission in the rat spinal cord. Neurosci Lett. 1993 Jul 23;157(2):149–152. doi: 10.1016/0304-3940(93)90724-y. [DOI] [PubMed] [Google Scholar]
- Choca J. I., Green R. D., Proudfit H. K. Adenosine A1 and A2 receptors of the substantia gelatinosa are located predominantly on intrinsic neurons: an autoradiography study. J Pharmacol Exp Ther. 1988 Nov;247(2):757–764. [PubMed] [Google Scholar]
- Craig C. G., White T. D. Low-level N-methyl-D-aspartate receptor activation provides a purinergic inhibitory threshold against further N-methyl-D-aspartate-mediated neurotransmission in the cortex. J Pharmacol Exp Ther. 1992 Mar;260(3):1278–1284. [PubMed] [Google Scholar]
- Craig C. G., White T. D. N-methyl-D-aspartate- and non-N-methyl-D-aspartate-evoked adenosine release from rat cortical slices: distinct purinergic sources and mechanisms of release. J Neurochem. 1993 Mar;60(3):1073–1080. doi: 10.1111/j.1471-4159.1993.tb03256.x. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Lodge D. Evidence for involvement of N-methylaspartate receptors in 'wind-up' of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987 Oct 27;424(2):402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Sullivan A. F. Electrophysiological studies on the effects of intrathecal morphine on nociceptive neurones in the rat dorsal horn. Pain. 1986 Feb;24(2):211–222. doi: 10.1016/0304-3959(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Sullivan A. F. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987 Aug;26(8):1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Sullivan A. F., McQuay H. J. Intrathecal etorphine, fentanyl and buprenorphine on spinal nociceptive neurones in the rat. Pain. 1990 Aug;42(2):227–234. doi: 10.1016/0304-3959(90)91166-G. [DOI] [PubMed] [Google Scholar]
- Dubner R., Ruda M. A. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992 Mar;15(3):96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Geiger J. D., LaBella F. S., Nagy J. I. Characterization and localization of adenosine receptors in rat spinal cord. J Neurosci. 1984 Sep;4(9):2303–2310. doi: 10.1523/JNEUROSCI.04-09-02303.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Synder S. H. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982 Sep;2(9):1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTON F. A., HOLTON P. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J Physiol. 1954 Oct 28;126(1):124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTON P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol. 1959 Mar 12;145(3):494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. On the nature of vibration receptors in the hind limb of the cat. J Physiol. 1961 Jan;155:175–186. doi: 10.1113/jphysiol.1961.sp006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J. E., Sullivan A. F., Dickenson A. H. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990 Jun 4;518(1-2):218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- Hoehn K., White T. D. Role of excitatory amino acid receptors in K+- and glutamate-evoked release of endogenous adenosine from rat cortical slices. J Neurochem. 1990 Jan;54(1):256–265. doi: 10.1111/j.1471-4159.1990.tb13309.x. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J. C., Singh L. Role of excitatory amino acid receptors in the mediation of the nociceptive response to formalin in the rat. Neurosci Lett. 1994 Jun 20;174(2):217–221. doi: 10.1016/0304-3940(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Jacobson K. A., van Galen P. J., Williams M. Adenosine receptors: pharmacology, structure-activity relationships, and therapeutic potential. J Med Chem. 1992 Feb 7;35(3):407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsten R., Gordh T., Jr, Hartvig P., Post C. Effects of intrathecal injection of the adenosine receptor agonists R-phenylisopropyl-adenosine and N-ethylcarboxamide-adenosine on nociception and motor function in the rat. Anesth Analg. 1990 Jul;71(1):60–64. doi: 10.1213/00000539-199007000-00010. [DOI] [PubMed] [Google Scholar]
- Li J., Perl E. R. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J Neurophysiol. 1994 Oct;72(4):1611–1621. doi: 10.1152/jn.1994.72.4.1611. [DOI] [PubMed] [Google Scholar]
- Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci. 1994 Aug;15(8):298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Malmberg A. B., Yaksh T. L. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiology. 1993 Aug;79(2):270–281. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- Manzoni O. J., Manabe T., Nicoll R. A. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994 Sep 30;265(5181):2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Lewin G. R., Wall P. D. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993 Aug;3(4):602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Buss M., LaBella L. A., Daddona P. E. Immunohistochemical localization of adenosine deaminase in primary afferent neurons of the rat. Neurosci Lett. 1984 Jul 27;48(2):133–138. doi: 10.1016/0304-3940(84)90008-9. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Daddona P. E. Anatomical and cytochemical relationships of adenosine deaminase-containing primary afferent neurons in the rat. Neuroscience. 1985 Jul;15(3):799–813. doi: 10.1016/0306-4522(85)90079-x. [DOI] [PubMed] [Google Scholar]
- Paalzow G., Paalzow L. The effects of caffeine and theophylline on nociceptive stimulation in the rat. Acta Pharmacol Toxicol (Copenh) 1973;32(1):22–32. doi: 10.1111/j.1600-0773.1973.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Salter M. W., De Koninck Y., Henry J. L. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog Neurobiol. 1993 Aug;41(2):125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- Salter M. W., Henry J. L. Effects of adenosine 5'-monophosphate and adenosine 5'-triphosphate on functionally identified units in the cat spinal dorsal horn. Evidence for a differential effect of adenosine 5'-triphosphate on nociceptive vs non-nociceptive units. Neuroscience. 1985 Jul;15(3):815–825. doi: 10.1016/0306-4522(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Salter M. W., Henry J. L. Evidence that adenosine mediates the depression of spinal dorsal horn neurons induced by peripheral vibration in the cat. Neuroscience. 1987 Aug;22(2):631–650. doi: 10.1016/0306-4522(87)90359-9. [DOI] [PubMed] [Google Scholar]
- Sawynok J., Sweeney M. I. The role of purines in nociception. Neuroscience. 1989;32(3):557–569. doi: 10.1016/0306-4522(89)90278-9. [DOI] [PubMed] [Google Scholar]
- Sosnowski M., Stevens C. W., Yaksh T. L. Assessment of the role of A1/A2 adenosine receptors mediating the purine antinociception, motor and autonomic function in the rat spinal cord. J Pharmacol Exp Ther. 1989 Sep;250(3):915–922. [PubMed] [Google Scholar]
- Sosnowski M., Yaksh T. L. Role of spinal adenosine receptors in modulating the hyperesthesia produced by spinal glycine receptor antagonism. Anesth Analg. 1989 Nov;69(5):587–592. [PubMed] [Google Scholar]
- Sweeney M. I., White T. D., Sawynok J. Morphine, capsaicin and K+ release purines from capsaicin-sensitive primary afferent nerve terminals in the spinal cord. J Pharmacol Exp Ther. 1989 Jan;248(1):447–454. [PubMed] [Google Scholar]
- Yaksh T. L. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989 Apr;37(1):111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]


