Abstract
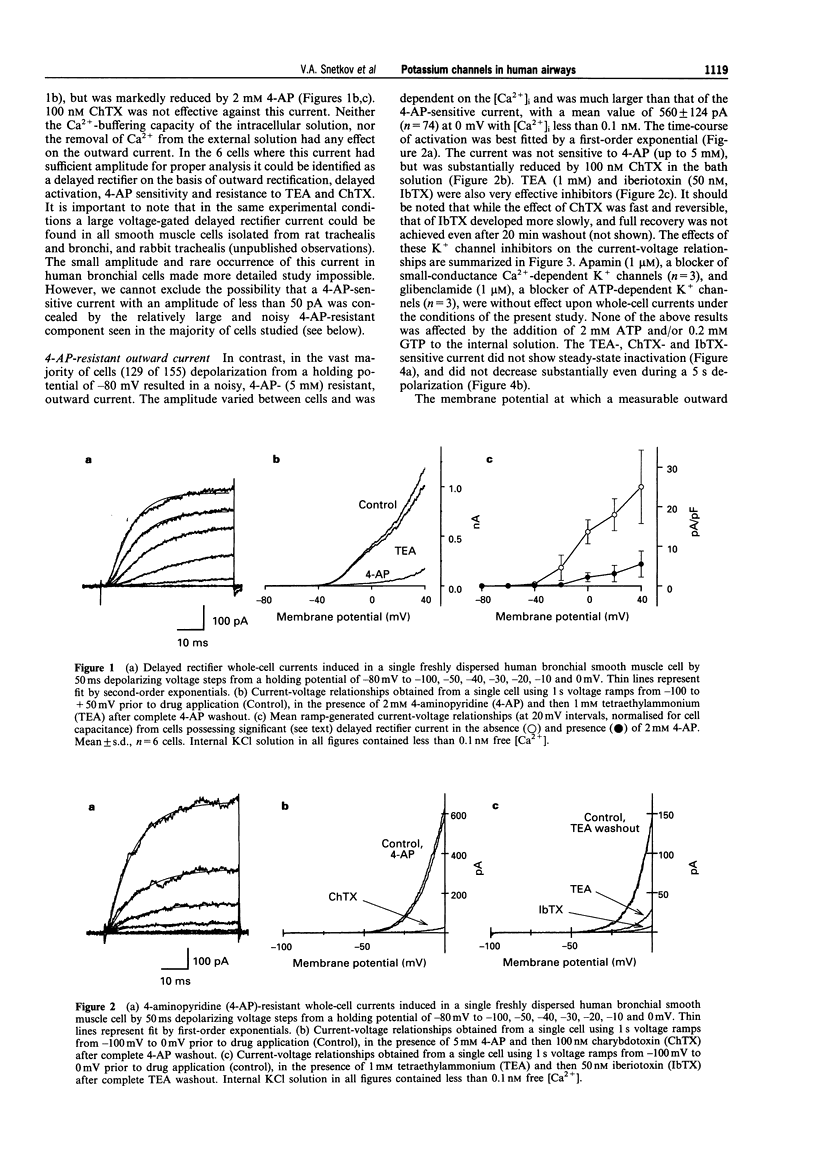
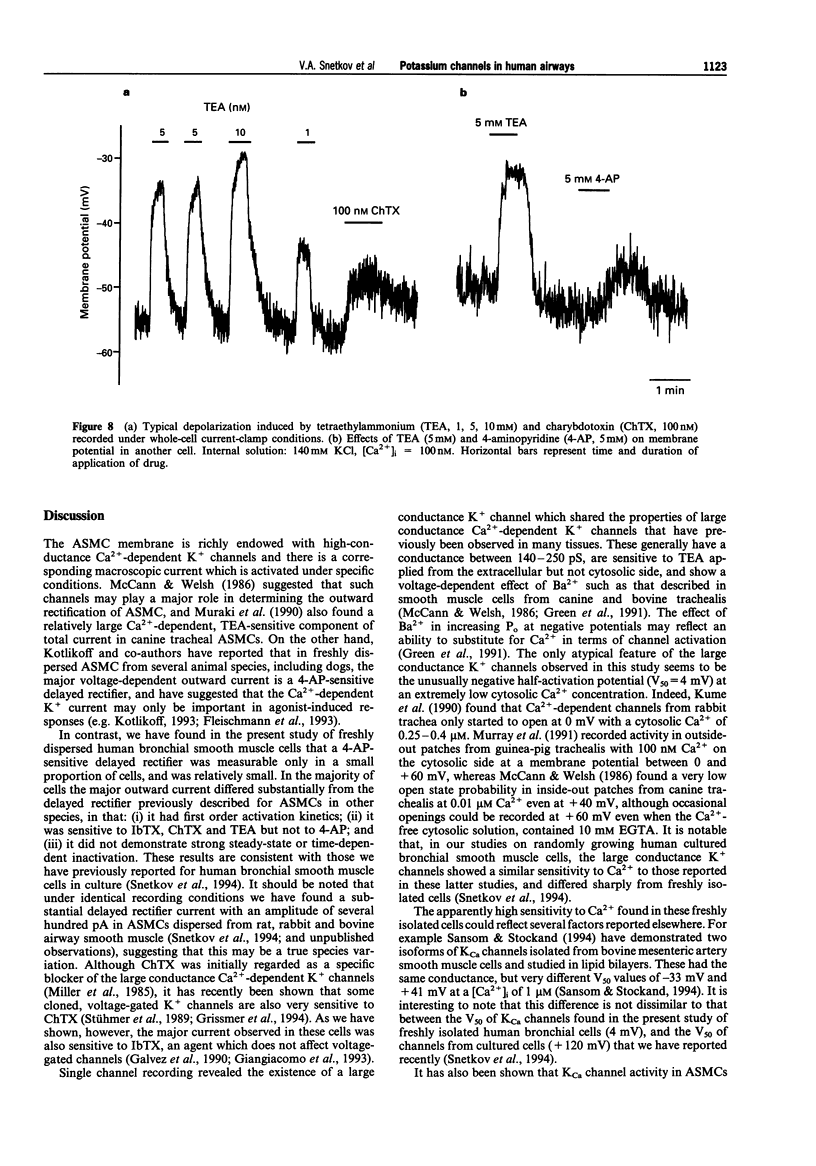
1. K+ currents were studied in smooth muscle cells enzymatically dissociated from human bronchi, by use of the patch-clamp technique. 2. In whole-cell recordings a depolarization-induced, 4-aminopyridine (4-AP)-sensitive current was observed in only 26 of 155 cells, and in 20 of these 26 cells its amplitude at a test potential of 0 mV was less than 100 pA. 3. In the majority of cells depolarization to -40 mV or more positive potentials induced a noisy outward current which activated within milliseconds and showed almost no inactivation even during a 5 s depolarizing voltage step. This current was insensitive to 4-AP (up to 5 mM) but was strongly inhibited in the presence of tetraethylammonium (TEA, 1 mM), charybdotoxin (ChTX, 100 nM) or iberiotoxin (IbTX, 50 nM) in the bath. The same current was also recorded by the nystatin-perforated patch technique. 4. Single channels with a conductance of about 210 pS were recorded in cell-attached patch, inside-out patch, outside-out patch and whole-cell recording configurations. Channel open state probability in inside-out patches was 0.5 at a membrane potential of 4 +/- 14 mV (mean +/- s.d., n = 13) mV even with a free Ca2+ concentration on the cytosolic side of the patch of less than 0.1 nM. Open state probability increased with depolarization and internal Ca2+ concentration. Single channels could be reversibly blocked by externally applied TEA, ChTX and IbTX. 5. In current-clamp recordings with 100 nM free Ca2+ in the intracellular solution both TEA and ChTX caused substantial concentration-dependent depolarization.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
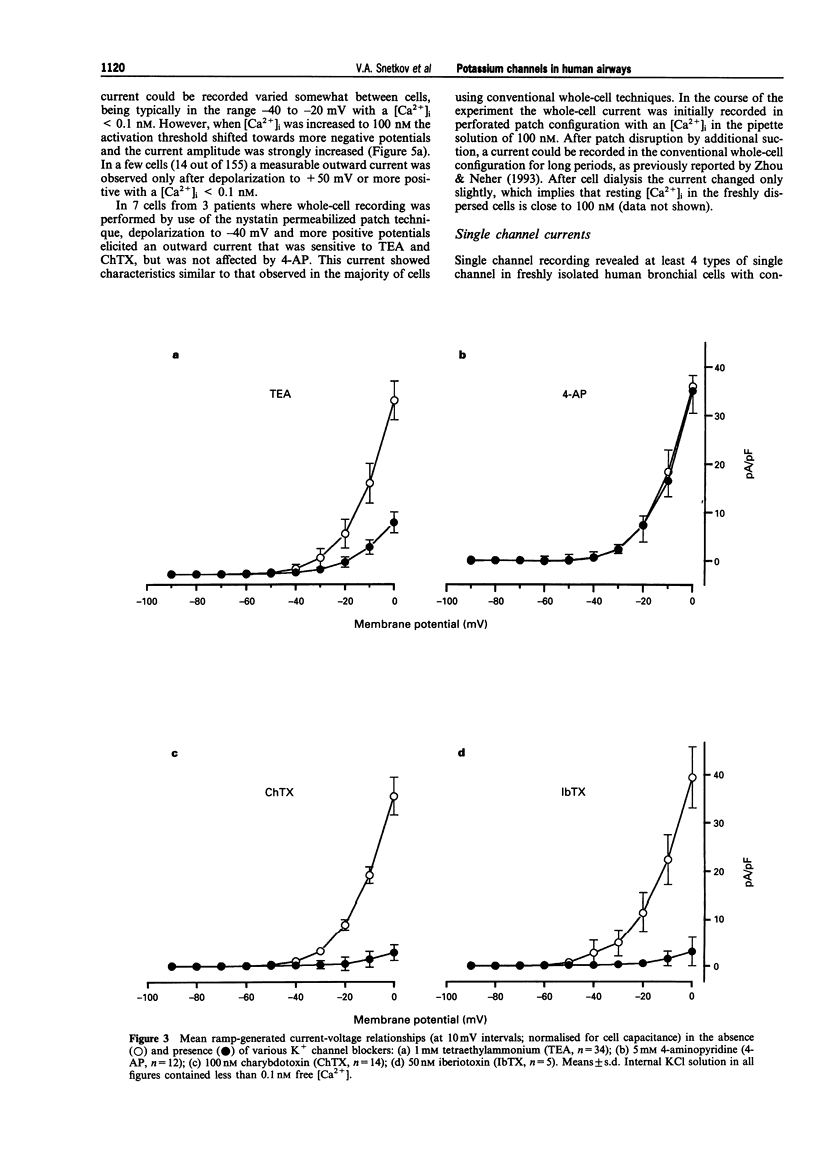
PDF

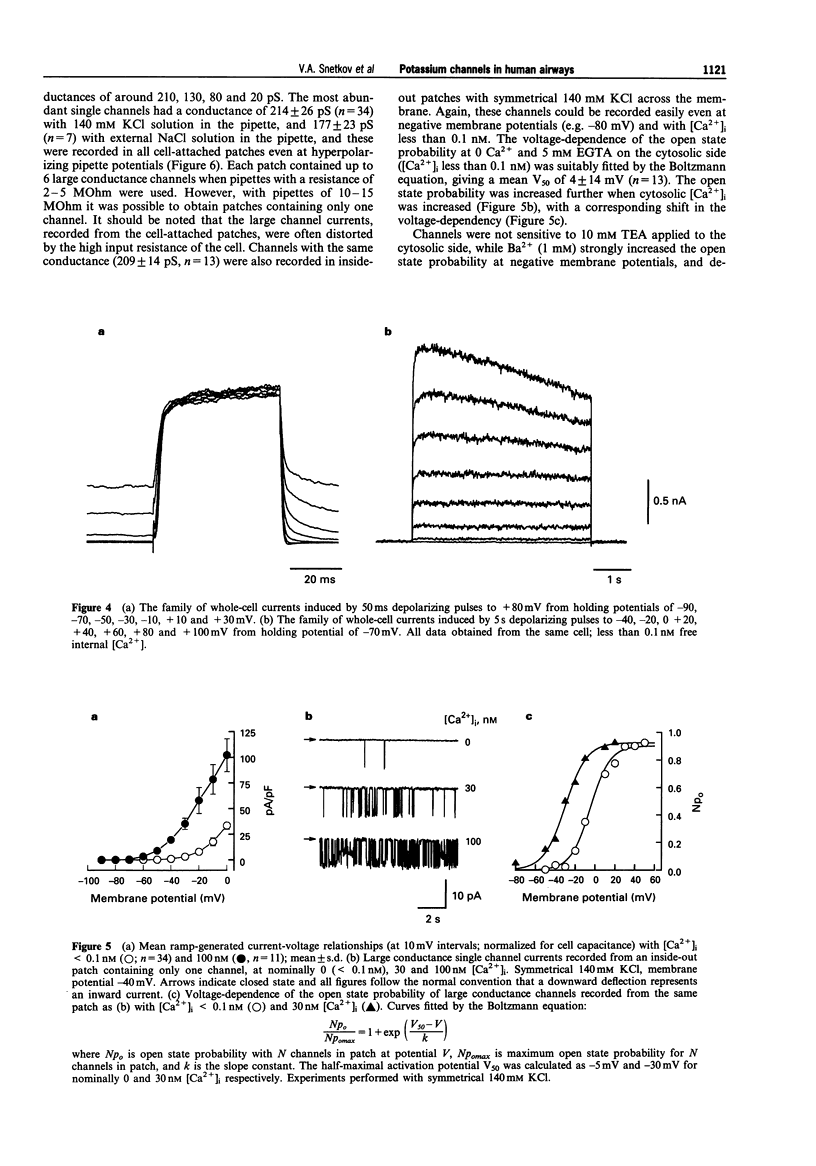
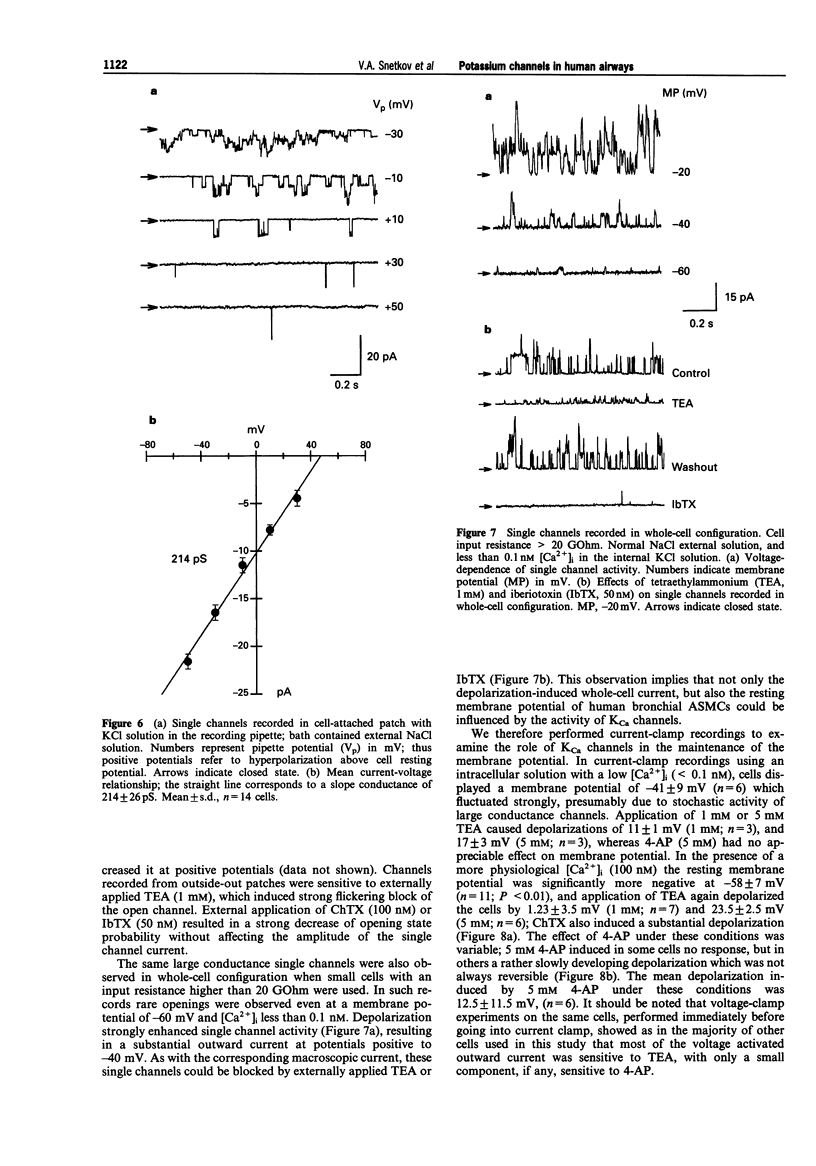


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano M., Masuzawa-Ito K., Matsuda T. Charybdotoxin-sensitive K+ channels regulate the myogenic tone in the resting state of arteries from spontaneously hypertensive rats. Br J Pharmacol. 1993 Jan;108(1):214–222. doi: 10.1111/j.1476-5381.1993.tb13465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fleischmann B. K., Washabau R. J., Kotlikoff M. I. Control of resting membrane potential by delayed rectifier potassium currents in ferret airway smooth muscle cells. J Physiol. 1993 Sep;469:625–638. doi: 10.1113/jphysiol.1993.sp019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furspan P. B., Webb R. C. Potassium channels and vascular reactivity in genetically hypertensive rats. Hypertension. 1990 Jun;15(6 Pt 2):687–691. doi: 10.1161/01.hyp.15.6.687. [DOI] [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Giangiacomo K. M., Sugg E. E., Garcia-Calvo M., Leonard R. J., McManus O. B., Kaczorowski G. J., Garcia M. L. Synthetic charybdotoxin-iberiotoxin chimeric peptides define toxin binding sites on calcium-activated and voltage-dependent potassium channels. Biochemistry. 1993 Mar 9;32(9):2363–2370. doi: 10.1021/bi00060a030. [DOI] [PubMed] [Google Scholar]
- Green K. A., Foster R. W., Small R. C. A patch-clamp study of K(+)-channel activity in bovine isolated tracheal smooth muscle cells. Br J Pharmacol. 1991 Apr;102(4):871–878. doi: 10.1111/j.1476-5381.1991.tb12269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Nguyen A. N., Aiyar J., Hanson D. C., Mather R. J., Gutman G. A., Karmilowicz M. J., Auperin D. D., Chandy K. G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994 Jun;45(6):1227–1234. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan M. S., Jager L. P., Daniel E. E., Garfield R. E. Effects of 4-aminopyridine and tetraethylammonium chloride on the electrical activity and cable properties of canine tracheal smooth muscle. J Pharmacol Exp Ther. 1983 Dec;227(3):706–715. [PubMed] [Google Scholar]
- Kotlikoff M. I. Potassium channels in airway smooth muscle: a tale of two channels. Pharmacol Ther. 1993;58(1):1–12. doi: 10.1016/0163-7258(93)90064-k. [DOI] [PubMed] [Google Scholar]
- Kume H., Hall I. P., Washabau R. J., Takagi K., Kotlikoff M. I. Beta-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994 Jan;93(1):371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takagi K., Satake T., Tokuno H., Tomita T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J Physiol. 1990 May;424:445–457. doi: 10.1113/jphysiol.1990.sp018076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Miura M., Belvisi M. G., Stretton C. D., Yacoub M. H., Barnes P. J. Role of potassium channels in bronchodilator responses in human airways. Am Rev Respir Dis. 1992 Jul;146(1):132–136. doi: 10.1164/ajrccm/146.1.132. [DOI] [PubMed] [Google Scholar]
- Muraki K., Imaizumi Y., Kojima T., Kawai T., Watanabe M. Effects of tetraethylammonium and 4-aminopyridine on outward currents and excitability in canine tracheal smooth muscle cells. Br J Pharmacol. 1990 Jul;100(3):507–515. doi: 10.1111/j.1476-5381.1990.tb15838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. A., Berry J. L., Cook S. J., Foster R. W., Green K. A., Small R. C. Guinea-pig isolated trachealis: the effects of charybdotoxin on mechanical activity, membrane potential changes and the activity of plasmalemmal K(+)-channels. Br J Pharmacol. 1991 Jul;103(3):1814–1818. doi: 10.1111/j.1476-5381.1991.tb09868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. K., Fleischmann B. K., Kotlikoff M. I. Receptor-activated Ca influx in human airway smooth muscle: use of Ca imaging and perforated patch-clamp techniques. Am J Physiol. 1993 Feb;264(2 Pt 1):C485–C490. doi: 10.1152/ajpcell.1993.264.2.C485. [DOI] [PubMed] [Google Scholar]
- Murray R. K., Kotlikoff M. I. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991 Apr;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. E., Schubert R., Hescheler J., Nelson M. T. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993 Jul;265(1 Pt 1):C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Saunders H. M., Farley J. M. Spontaneous transient outward currents and Ca(++)-activated K+ channels in swine tracheal smooth muscle cells. J Pharmacol Exp Ther. 1991 Jun;257(3):1114–1120. [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Kurtz G., Garcia M. L., Kaczorowski G. J. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea pig smooth muscle tissues. J Pharmacol Exp Ther. 1991 Oct;259(1):439–443. [PubMed] [Google Scholar]


