Abstract
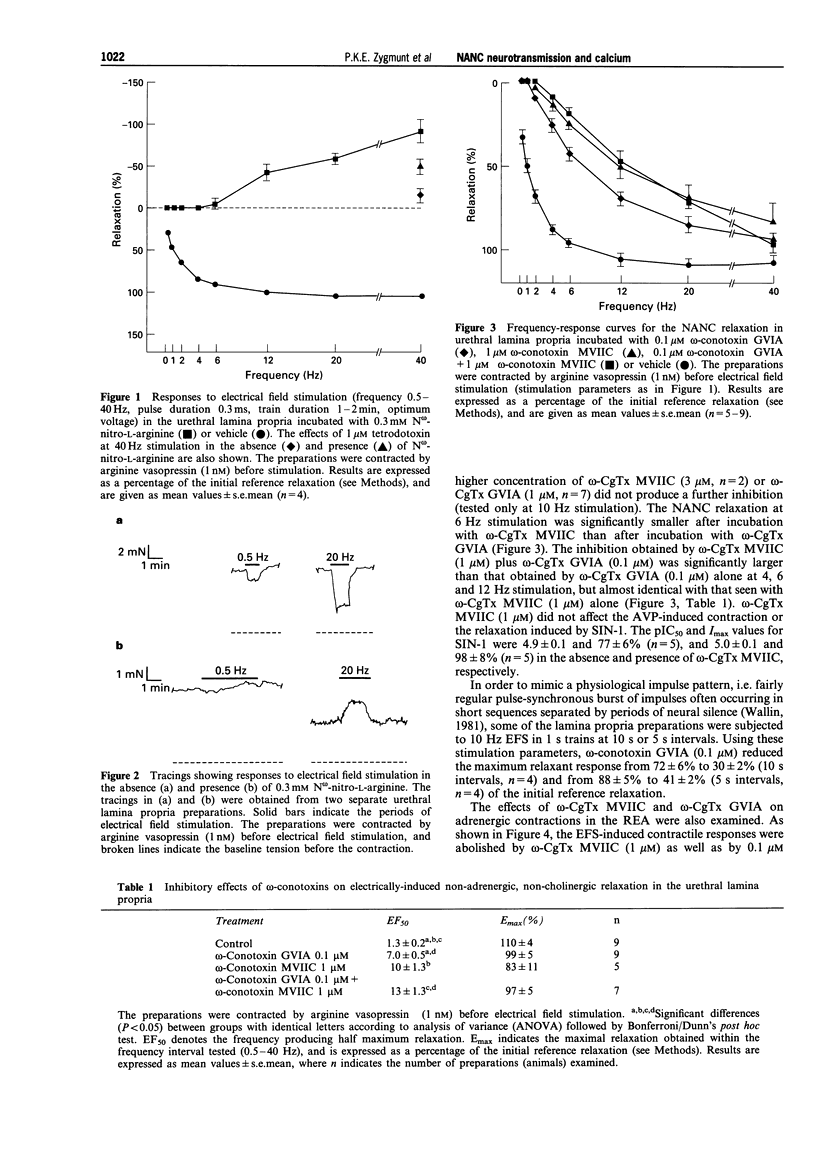
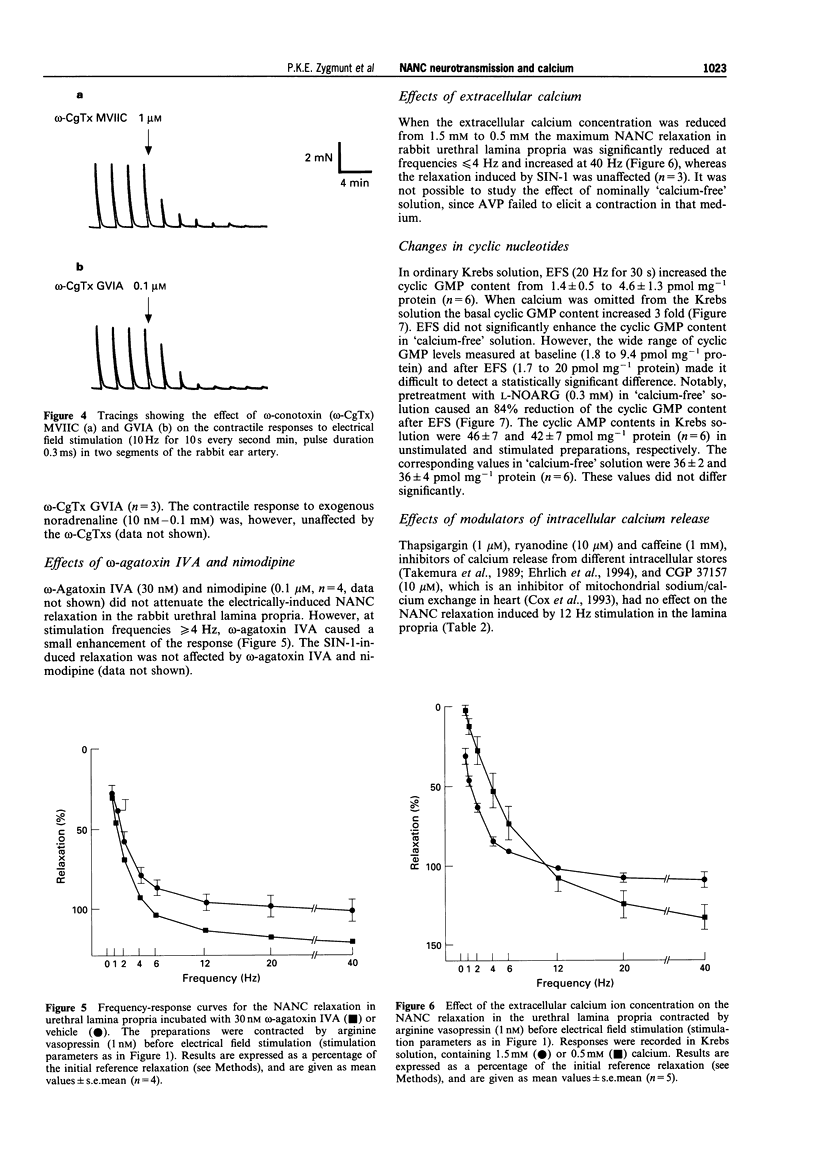
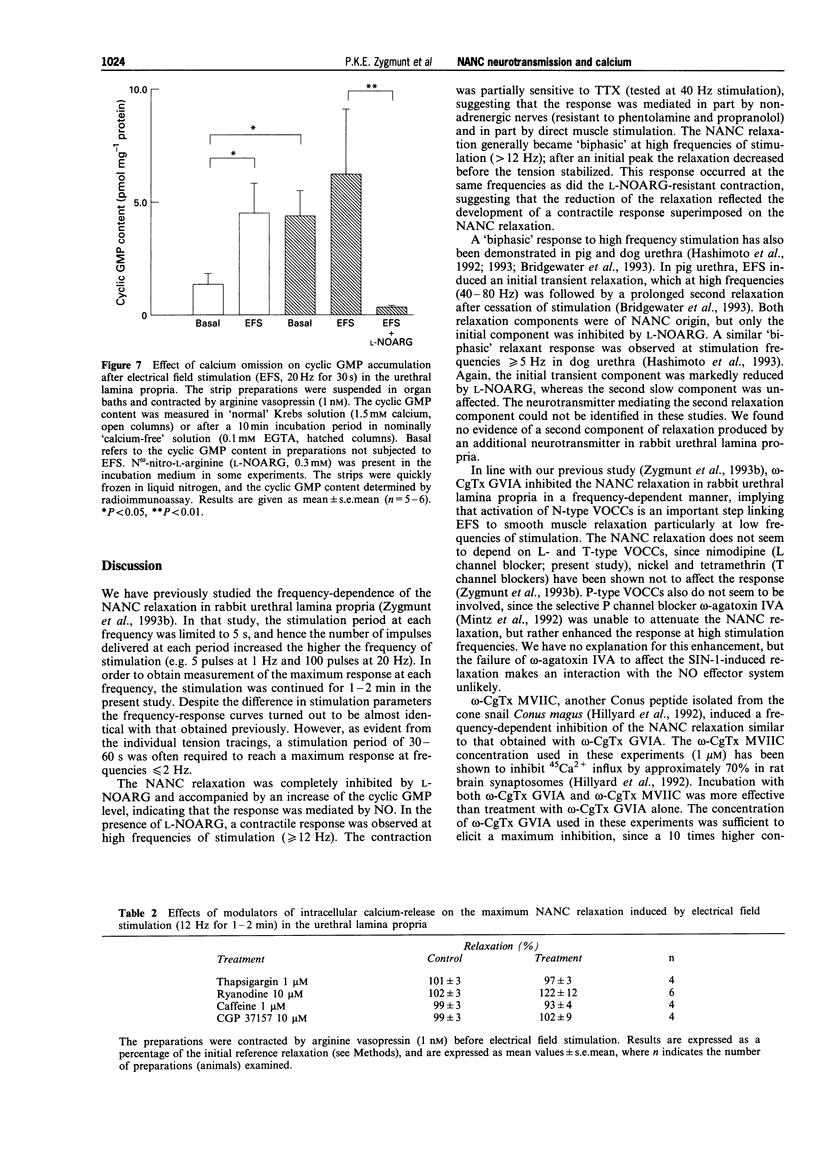
1. Electrical field stimulation (EFS) of the rabbit urethral lamina propria elicited a frequency-dependent non-adrenergic, non-cholinergic (NANC) relaxation, which was abolished by N omega-nitro-L-arginine (L-NOARG). 2. omega-Conotoxin GVIA, a selective blocker of N-type voltage-operated calcium channels (VOCCs), and omega-conotoxin MVIIC (blocker of N- and Q-type VOCCs) inhibited the NANC relaxation, and the inhibition was inversely related to the frequency of stimulation. Combined, the two toxins were more effective than omega-contoxin GVIA alone. 3. The relaxation induced by the nitric oxide (NO) donor, 3-morpholino-sydnonimine (SIN-1) was not affected by omega-conotoxin MVIIC. 4. omega-Agatoxin IVA (blocker of P-type VOCCs) did not attenuate the NANC relaxation. 5. Reduction of the calcium concentration from 1.5 to 0.5 mM reduced the NANC relaxation at low but not at high frequencies of stimulation; the relaxation induced by SIN-1 was not affected. 6. EFS (20 Hz, 30 s) increased the cyclic GMP level 3 fold in normal Krebs solutions, but was unable to enhance significantly the cyclic GMP level after calcium omission. L-NOARG reduced the cyclic GMP content in 'calcium-free' medium, indicating an ongoing NO synthesis that was independent of extracellular calcium. 7. Caffeine, ryanodine and thapsigargin (inhibitors of sarcoplasmic calcium release), and CGP 37157 (inhibitor of mitochondrial sodium/calcium exchange) had no effect on the NANC relaxation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993 Sep;45(3):253–308. [PubMed] [Google Scholar]
- Belai A., Ralevic V., Burnstock G. VIP release from enteric nerves is independent of extracellular calcium. Regul Pept. 1987 Oct;19(1-2):79–89. doi: 10.1016/0167-0115(87)90077-2. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bridgewater M., MacNeil H. F., Brading A. F. Regulation of tone in pig urethral smooth muscle. J Urol. 1993 Jul;150(1):223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- Cox D. A., Conforti L., Sperelakis N., Matlib M. A. Selectivity of inhibition of Na(+)-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J Cardiovasc Pharmacol. 1993 Apr;21(4):595–599. doi: 10.1097/00005344-199304000-00013. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Kaftan E., Bezprozvannaya S., Bezprozvanny I. The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci. 1994 May;15(5):145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Pascual A., Costa G., Garcia-Sacristan A., Andersson K. E. Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol Scand. 1991 Apr;141(4):531–539. doi: 10.1111/j.1748-1716.1991.tb09114.x. [DOI] [PubMed] [Google Scholar]
- Grantham C. J., Bowman D., Bath C. P., Bell D. C., Bleakman D. Omega-conotoxin MVIIC reversibly inhibits a human N-type calcium channel and calcium influx into chick synaptosomes. Neuropharmacology. 1994 Feb;33(2):255–258. doi: 10.1016/0028-3908(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Kigoshi S., Muramatsu I. Neurogenic responses of urethra isolated from the dog. Eur J Pharmacol. 1992 Mar 17;213(1):117–123. doi: 10.1016/0014-2999(92)90240-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Kigoshi S., Muramatsu I. Nitric oxide-dependent and -independent neurogenic relaxation of isolated dog urethra. Eur J Pharmacol. 1993 Feb 9;231(2):209–214. doi: 10.1016/0014-2999(93)90451-m. [DOI] [PubMed] [Google Scholar]
- Hillyard D. R., Monje V. D., Mintz I. M., Bean B. P., Nadasdi L., Ramachandran J., Miljanich G., Azimi-Zoonooz A., McIntosh J. M., Cruz L. J. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992 Jul;9(1):69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Kostka P., Jang E., Watson E. G., Stewart J. L., Daniel E. E. Nitric oxide synthase in the autonomic nervous system of canine ileum. J Pharmacol Exp Ther. 1993 Jan;264(1):234–239. [PubMed] [Google Scholar]
- Ludbrook J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc Res. 1994 Mar;28(3):303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- Minchin M. C. The role of Ca2+ in the protoveratrine-induced release of gamma-aminobutyrate from rat brain slices. Biochem J. 1980 Aug 15;190(2):333–339. doi: 10.1042/bj1900333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Murad F., Forstermann U., Nakane M., Schmidt H., Pollock J., Sheng H., Matsumoto T., Warner T., Mitchell J., Tracey R. The nitric oxide-cyclic GMP signal transduction pathway in vascular smooth muscle preparations and other tissues. Jpn J Pharmacol. 1992;58 (Suppl 2):150P–157P. [PubMed] [Google Scholar]
- Sandoval M. E. Sodium-dependent efflux of [3H]GABA from synaptosomes probably related to mitochondrial calcium mobilization. J Neurochem. 1980 Oct;35(4):915–921. doi: 10.1111/j.1471-4159.1980.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer A. N., Mulder A. H. [3H]noradrenaline release from brain slices induced by an increase in the intracellular sodium concentration: role of intracellular calcium stores. J Neurochem. 1983 Mar;40(3):615–621. doi: 10.1111/j.1471-4159.1983.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Graham A. Role of nitric oxide in the autonomic innervation of smooth muscle. J Auton Pharmacol. 1992 Dec;12(6):445–456. doi: 10.1111/j.1474-8673.1992.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992 Jul 24;257(5069):494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Wheeler D. B., Randall A., Tsien R. W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994 Apr 1;264(5155):107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. K., Persson K., Alm P., Larsson B., Andersson K. E. The L-arginine/nitric oxide pathway in the rabbit urethral lamina propria. Acta Physiol Scand. 1993 Aug;148(4):431–439. doi: 10.1111/j.1748-1716.1993.tb09579.x. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. M., Högestätt E. D. Calcium channels at the adrenergic neuroeffector junction in the rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jun;347(6):617–623. doi: 10.1007/BF00166944. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. M., Zygmunt P. K., Högestätt E. D., Andersson K. E. Effects of omega-conotoxin on adrenergic, cholinergic and NANC neurotransmission in the rabbit urethra and detrusor. Br J Pharmacol. 1993 Dec;110(4):1285–1290. doi: 10.1111/j.1476-5381.1993.tb13957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


