Abstract
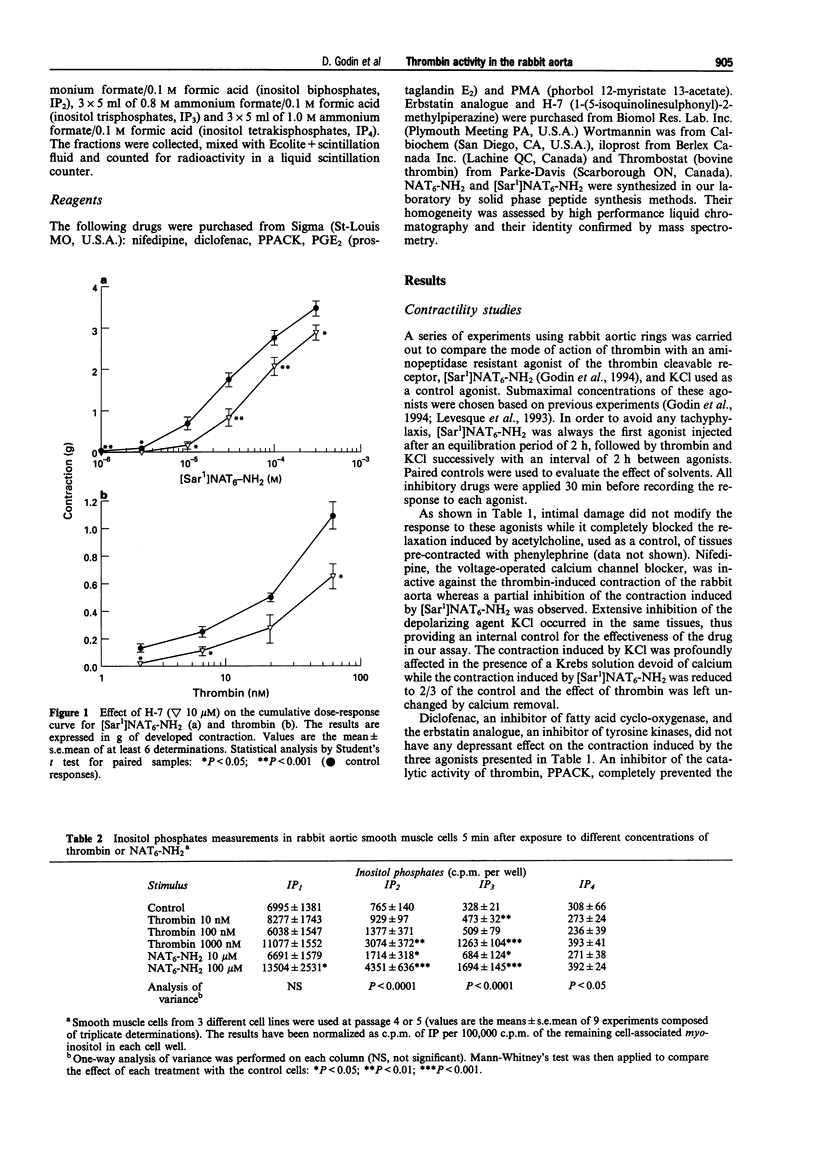
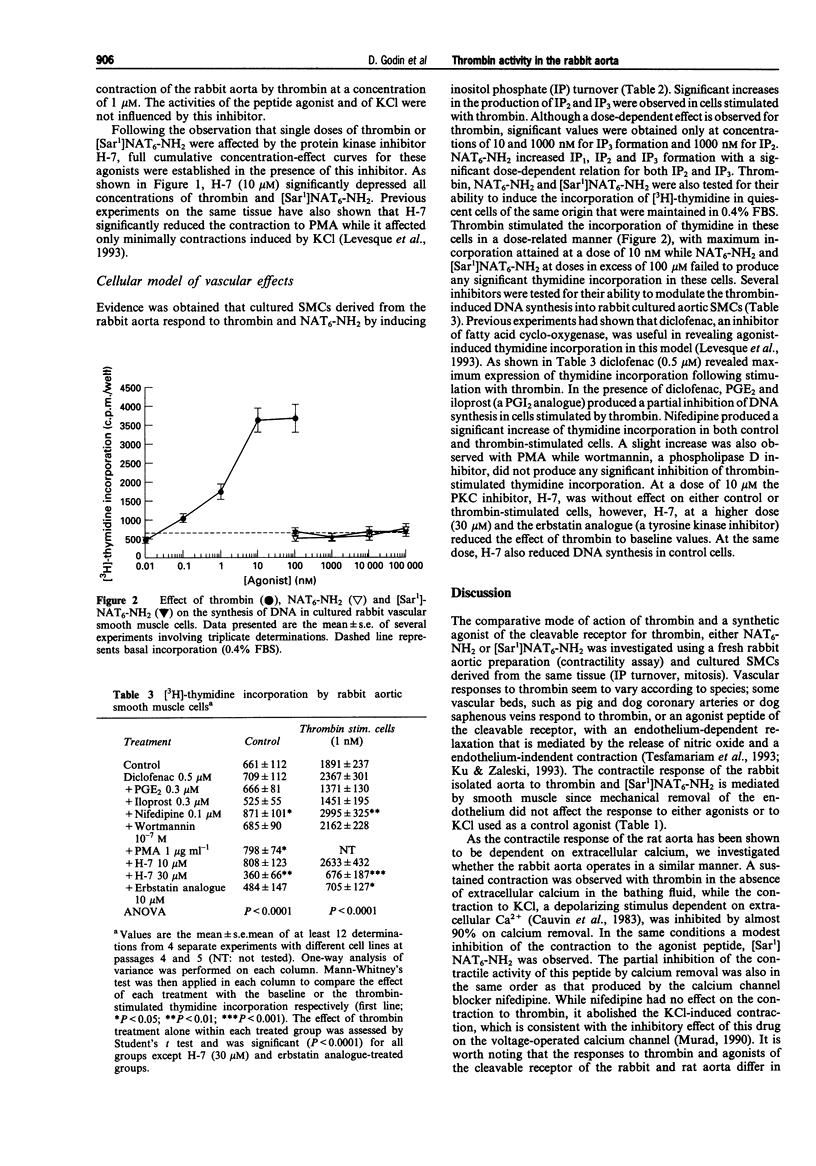
1. Thrombin is a vasoactive protease that elicits the contraction of the rabbit aorta by activating a G-protein coupled receptor through cleavage of its N-terminal extracellular domain. Synthetic peptides corresponding to the newly exposed N-terminus, following thrombin cleavage, have been shown to reproduce some of the activities of thrombin in the rabbit aorta. 2. Intracellular pathways involved in the contractile response of the rabbit aorta to thrombin and synthetic peptides were examined by use of a series of inhibitors. A similar method was applied to characterize the mitogenic effect of thrombin on cultured smooth muscle cells (SMCs) derived from the same tissue. 3. Results from this study indicate that the contractile response of the rabbit aorta to thrombin is dependent on the activation of protein kinase C (PKC) and independent of extracellular calcium. The contractile response to thrombin can be fully reproduced by peptide agonists related to the N-terminal receptor sequence. However, subtle differences seem to exist between the mechanism of the contractile effect of thrombin and of the synthetic peptides, as both PKC activation and extracellular calcium were found to participate in the contractile effect of the synthetic peptides. 4. In cultured SMCs, both thrombin and the synthetic peptides increased inositol phosphate turnover; however, only thrombin elicited a mitogenic effect, which occurs at thrombin concentrations well below those needed to increase inositol phosphate turnover significantly. Activation of a tyrosine kinase pathway is involved in the mitogenic effect of thrombin on aortic SMCs.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonaccio M. J., Normandin D., Serafino R., Moreland S. Effects of thrombin and thrombin receptor activating peptides on rat aortic vascular smooth muscle. J Pharmacol Exp Ther. 1993 Jul;266(1):125–132. [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Guillemette G., Catt K. J. Multiple pathways of inositol polyphosphate metabolism in angiotensin-stimulated adrenal glomerulosa cells. J Biol Chem. 1988 Mar 25;263(9):4083–4091. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthillier J., Deblois D., Marceau F. Studies on the induction of pharmacological responses to des-Arg9-bradykinin in vitro and in vivo. Br J Pharmacol. 1987 Oct;92(2):257–264. doi: 10.1111/j.1476-5381.1987.tb11319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauvin C., Loutzenhiser R., Van Breemen C. Mechanisms of calcium antagonist-induced vasodilation. Annu Rev Pharmacol Toxicol. 1983;23:373–396. doi: 10.1146/annurev.pa.23.040183.002105. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Vu T. K., Hung D. T., Wheaton V. I. Characterization of a functional thrombin receptor. Issues and opportunities. J Clin Invest. 1992 Feb;89(2):351–355. doi: 10.1172/JCI115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Regulation of thrombin generation and functions. Semin Thromb Hemost. 1988 Jul;14(3):234–240. doi: 10.1055/s-2007-1002783. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Godin D., Marceau F., Beaulé C., Rioux F., Drapeau G. Aminopeptidase modulation of the pharmacological responses to synthetic thrombin receptor agonists. Eur J Pharmacol. 1994 Mar 3;253(3):225–230. doi: 10.1016/0014-2999(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Guillemette G., Lamontagne S., Boulay G., Mouillac B. Differential effects of heparin on inositol 1,4,5-trisphosphate binding, metabolism, and calcium release activity in the bovine adrenal cortex. Mol Pharmacol. 1989 Mar;35(3):339–344. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Laniyonu A. A., Saifeddine M., Moore G. J. Role of the amino- and carboxyl-terminal domains of thrombin receptor-derived polypeptides in biological activity in vascular endothelium and gastric smooth muscle: evidence for receptor subtypes. Mol Pharmacol. 1993 Jun;43(6):921–930. [PubMed] [Google Scholar]
- Hui K. Y., Jakubowski J. A., Wyss V. L., Angleton E. L. Minimal sequence requirement of thrombin receptor agonist peptide. Biochem Biophys Res Commun. 1992 Apr 30;184(2):790–796. doi: 10.1016/0006-291x(92)90659-9. [DOI] [PubMed] [Google Scholar]
- Imoto M., Umezawa K., Isshiki K., Kunimoto S., Sawa T., Takeuchi T., Umezawa H. Kinetic studies of tyrosine kinase inhibition by erbstatin. J Antibiot (Tokyo) 1987 Oct;40(10):1471–1473. doi: 10.7164/antibiotics.40.1471. [DOI] [PubMed] [Google Scholar]
- Kettner C., Shaw E. D-Phe-Pro-ArgCH2C1-A selective affinity label for thrombin. Thromb Res. 1979;14(6):969–973. doi: 10.1016/0049-3848(79)90014-8. [DOI] [PubMed] [Google Scholar]
- Ku D. D., Zaleski J. K. Receptor mechanism of thrombin-induced endothelium-dependent and endothelium-independent coronary vascular effects in dogs. J Cardiovasc Pharmacol. 1993 Oct;22(4):609–616. doi: 10.1097/00005344-199310000-00015. [DOI] [PubMed] [Google Scholar]
- Levesque L., Drapeau G., Grose J. H., Rioux F., Marceau F. Vascular mode of action of kinin B1 receptors and development of a cellular model for the investigation of these receptors. Br J Pharmacol. 1993 Aug;109(4):1254–1262. doi: 10.1111/j.1476-5381.1993.tb13757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau F., Petitclerc E., DeBlois D., Pradelles P., Poubelle P. E. Human interleukin-1 induces a rapid relaxation of the rabbit isolated mesenteric artery. Br J Pharmacol. 1991 Jun;103(2):1367–1372. doi: 10.1111/j.1476-5381.1991.tb09795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt S., Emilsson K., Wahlestedt C., Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Allen G. T., Normandin D., Antonaccio M. J. Involvement of the "tethered ligand" receptor in thrombin-induced endothelium-mediated relaxations. Am J Physiol. 1993 Nov;265(5 Pt 2):H1744–H1749. doi: 10.1152/ajpheart.1993.265.5.H1744. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V., Van Obberghen-Schilling E., Rasmussen U. B., Pavirani A., Lecocq J. P., Pouysségur J. Synthetic alpha-thrombin receptor peptides activate G protein-coupled signaling pathways but are unable to induce mitogenesis. Mol Biol Cell. 1992 Jan;3(1):95–102. doi: 10.1091/mbc.3.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Weiss R. H., Maduri M. The mitogenic effect of thrombin in vascular smooth muscle cells is largely due to basic fibroblast growth factor. J Biol Chem. 1993 Mar 15;268(8):5724–5727. [PubMed] [Google Scholar]
- Weiss R. H., Nuccitelli R. Inhibition of tyrosine phosphorylation prevents thrombin-induced mitogenesis, but not intracellular free calcium release, in vascular smooth muscle cells. J Biol Chem. 1992 Mar 15;267(8):5608–5613. [PubMed] [Google Scholar]
- Yang S. G., Laniyonu A., Saifeddine M., Moore G. J., Hollenberg M. D. Actions of thrombin and thrombin receptor peptide analogues in gastric and aortic smooth muscle: development of bioassays for structure-activity studies. Life Sci. 1992;51(17):1325–1332. doi: 10.1016/0024-3205(92)90631-x. [DOI] [PubMed] [Google Scholar]
- deBlois D., Drapeau G., Petitclerc E., Marceau F. Synergism between the contractile effect of epidermal growth factor and that of des-Arg9-bradykinin or of alpha-thrombin in rabbit aortic rings. Br J Pharmacol. 1992 Apr;105(4):959–967. doi: 10.1111/j.1476-5381.1992.tb09085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


