Abstract
1 We have evaluated the selectivity of ketoprofen and two novel nonsteroidal anti-inflammatory drugs, N-[2-(cyclohexyloxy)-4-nitrophenyl]methanesulphonamide (NS-398) and 5-methanesulphonamido-6-(2,4-difluorothiophenyl)-1-indanone (L-745,337), in inhibiting the cyclo-oxygenase activity of prostaglandin endoperoxide synthase-2 (PGHS-2) vs PGHS-1 in human blood monocytes and platelets, respectively.
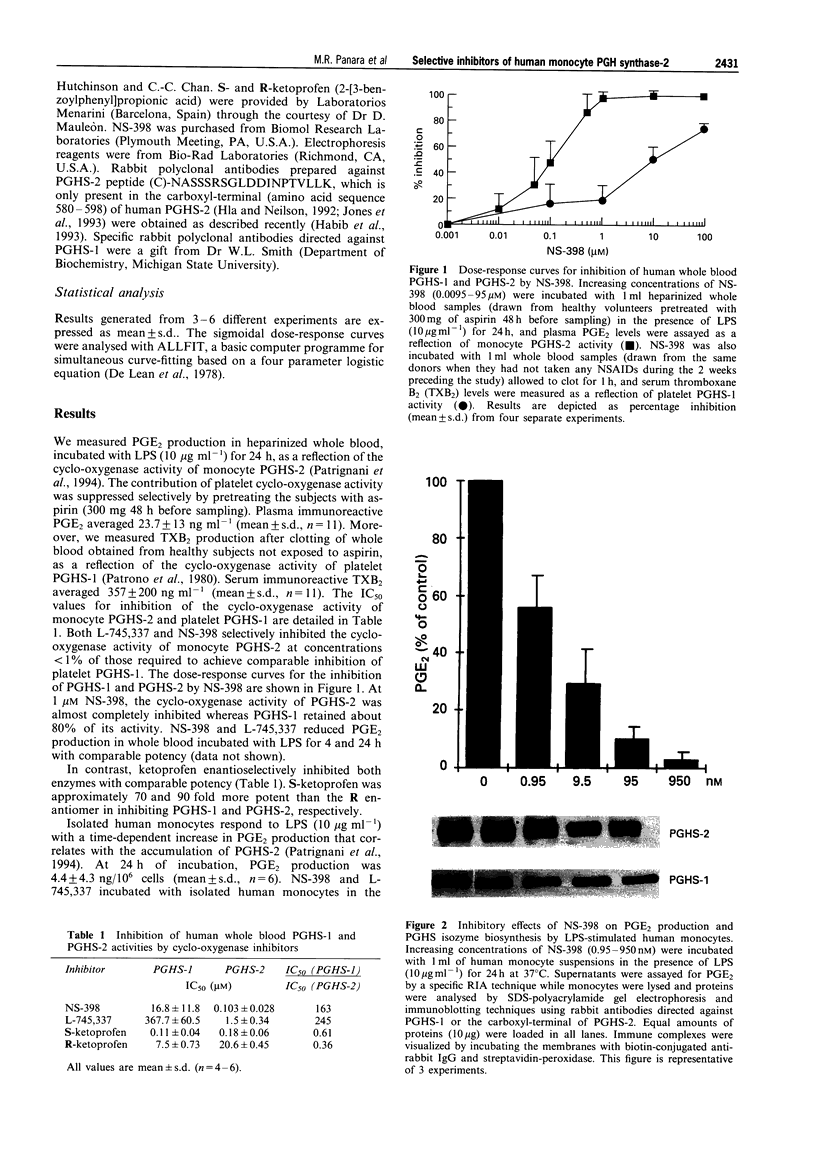
2 Heparinized whole blood samples were drawn from healthy volunteers pretreated with aspirin, 300 mg 48 h before sampling, to suppress the activity of platelet PGHS-1 and incubated at 37°C for 24 h with increasing concentrations of the test compounds in the presence of lipopolysaccharide (LPS, 10 μg ml-1). Immunoreactive PGE2 levels were measured in plasma by a specific radioimmunoassay as an index of the cyclo-oxygenase activity of LPS-induced monocyte PGHS-2.
3 The effects of the same inhibitors on platelet PGHS-1 activity were assessed by allowing whole blood samples, drawn from the same subjects in aspirin-free periods, to clot at 37°C for 1 h in the presence of the compounds and measuring immunoreactive thromboxane B2 (TXB2) levels in serum by a specific radioimmunoassay.
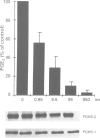
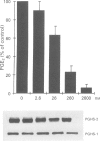
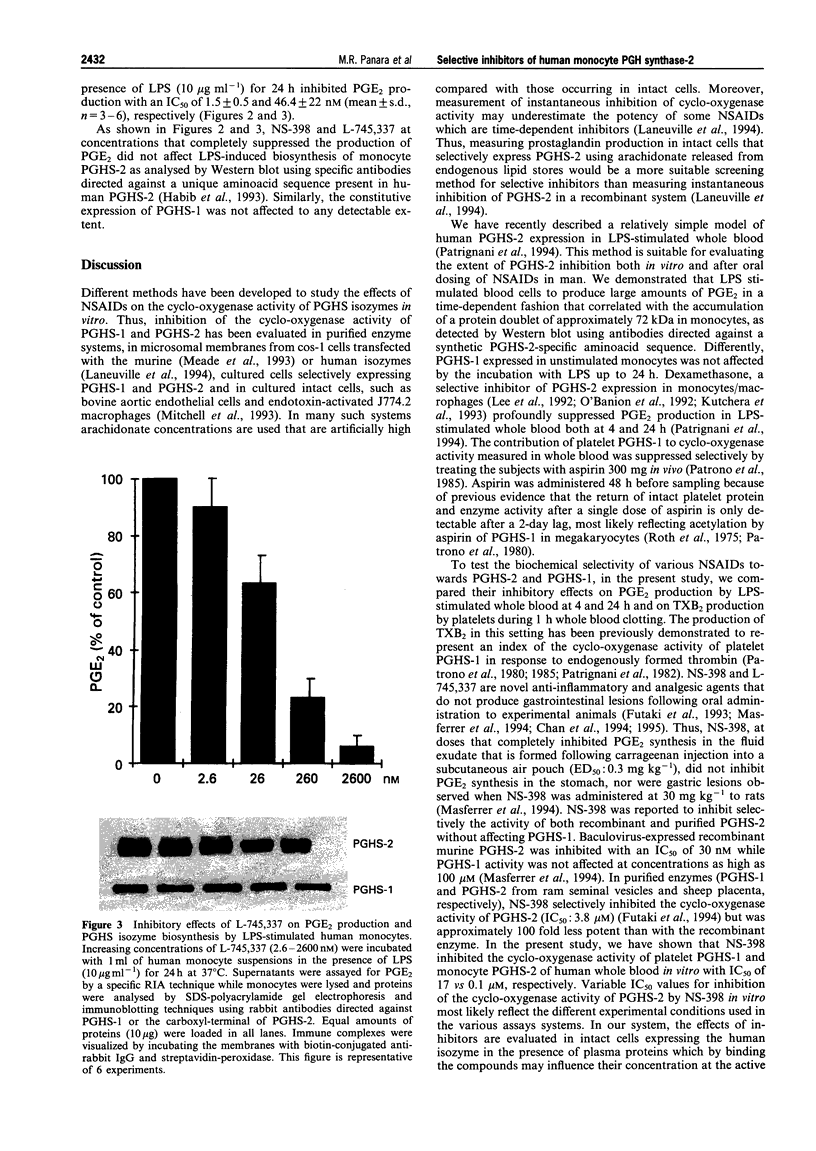
4 Under these experimental conditions, ketoprofen enantioselectively inhibited the cyclo-oxygenase activity of both PGHS-1 and PGHS-2 with equal potency (IC50 ratio: approx. 0.5 for both enantiomers), while L-745,337 and NS-398 achieved selective inhibition of monocyte PGHS-2 (IC50 ratio: >150). L-745,337 and NS-398 did not affect LPS-induced monocyte PGHS-2 biosynthesis to any detectable extent.
5 We conclude that L-745,337 and NS-398 are selective inhibitors of the cyclo-oxygenase activity of human monocyte PGHS-2. These compounds may provide adequate tools to test the contribution of this novel pathway of arachidonate metabolism to human inflammatory disease.
Keywords: Prostaglandin endoperoxide synthases; human blood monocytes; human platelets; L-745,337; NS-398; ketoprofen
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Ciabattoni G., Pugliese F., Spaldi M., Cinotti G. A., Patrono C. Radioimmunoassay measurement of prostaglandins E2 and F2alpha in human urine. J Endocrinol Invest. 1979 Apr-Jun;2(2):173–182. doi: 10.1007/BF03349310. [DOI] [PubMed] [Google Scholar]
- Copeland R. A., Williams J. M., Giannaras J., Nurnberg S., Covington M., Pinto D., Pick S., Trzaskos J. M. Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11202–11206. doi: 10.1073/pnas.91.23.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991 May 8;1083(2):121–134. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher B. S., Kujubu D. A., Perrin D. M., Herschman H. R. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J Biol Chem. 1992 Mar 5;267(7):4338–4344. [PubMed] [Google Scholar]
- Futaki N., Takahashi S., Yokoyama M., Arai I., Higuchi S., Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994 Jan;47(1):55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Futaki N., Yoshikawa K., Hamasaka Y., Arai I., Higuchi S., Iizuka H., Otomo S. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. Gen Pharmacol. 1993 Jan;24(1):105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- Habib A., Créminon C., Frobert Y., Grassi J., Pradelles P., Maclouf J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J Biol Chem. 1993 Nov 5;268(31):23448–23454. [PubMed] [Google Scholar]
- Hempel S. L., Monick M. M., Hunninghake G. W. Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J Clin Invest. 1994 Jan;93(1):391–396. doi: 10.1172/JCI116971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., Carlton D. P., McIntyre T. M., Zimmerman G. A., Prescott S. M. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993 Apr 25;268(12):9049–9054. [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Laneuville O., Breuer D. K., Dewitt D. L., Hla T., Funk C. D., Smith W. L. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 1994 Nov;271(2):927–934. [PubMed] [Google Scholar]
- Lee S. H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992 Dec 25;267(36):25934–25938. [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Manning P. T., Hauser S. D., Leahy K. M., Smith W. G., Isakson P. C., Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Mitchell J. A., Akarasereenont P., Thiemermann C., Flower R. J., Vane J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Banion M. K., Winn V. D., Young D. A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M. G., Chilton F. H., Huggins E. M., Jr, McCall C. E. Lipopolysaccharide priming of alveolar macrophages for enhanced synthesis of prostanoids involves induction of a novel prostaglandin H synthase. J Biol Chem. 1992 Jul 25;267(21):14547–14550. [PubMed] [Google Scholar]
- O'Sullivan M. G., Huggins E. M., Jr, Meade E. A., DeWitt D. L., McCall C. E. Lipopolysaccharide induces prostaglandin H synthase-2 in alveolar macrophages. Biochem Biophys Res Commun. 1992 Sep 16;187(2):1123–1127. doi: 10.1016/0006-291x(92)91313-f. [DOI] [PubMed] [Google Scholar]
- Patrignani P., Filabozzi P., Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982 Jun;69(6):1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrignani P., Panara M. R., Greco A., Fusco O., Natoli C., Iacobelli S., Cipollone F., Ganci A., Créminon C., Maclouf J. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J Pharmacol Exp Ther. 1994 Dec;271(3):1705–1712. [PubMed] [Google Scholar]
- Patrono C., Ciabattoni G., Patrignani P., Pugliese F., Filabozzi P., Catella F., Davì G., Forni L. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation. 1985 Dec;72(6):1177–1184. doi: 10.1161/01.cir.72.6.1177. [DOI] [PubMed] [Google Scholar]
- Patrono C., Ciabattoni G., Pinca E., Pugliese F., Castrucci G., De Salvo A., Satta M. A., Peskar B. A. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res. 1980 Feb 1;17(3-4):317–327. doi: 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vane J. Towards a better aspirin. Nature. 1994 Jan 20;367(6460):215–216. doi: 10.1038/367215a0. [DOI] [PubMed] [Google Scholar]