Abstract
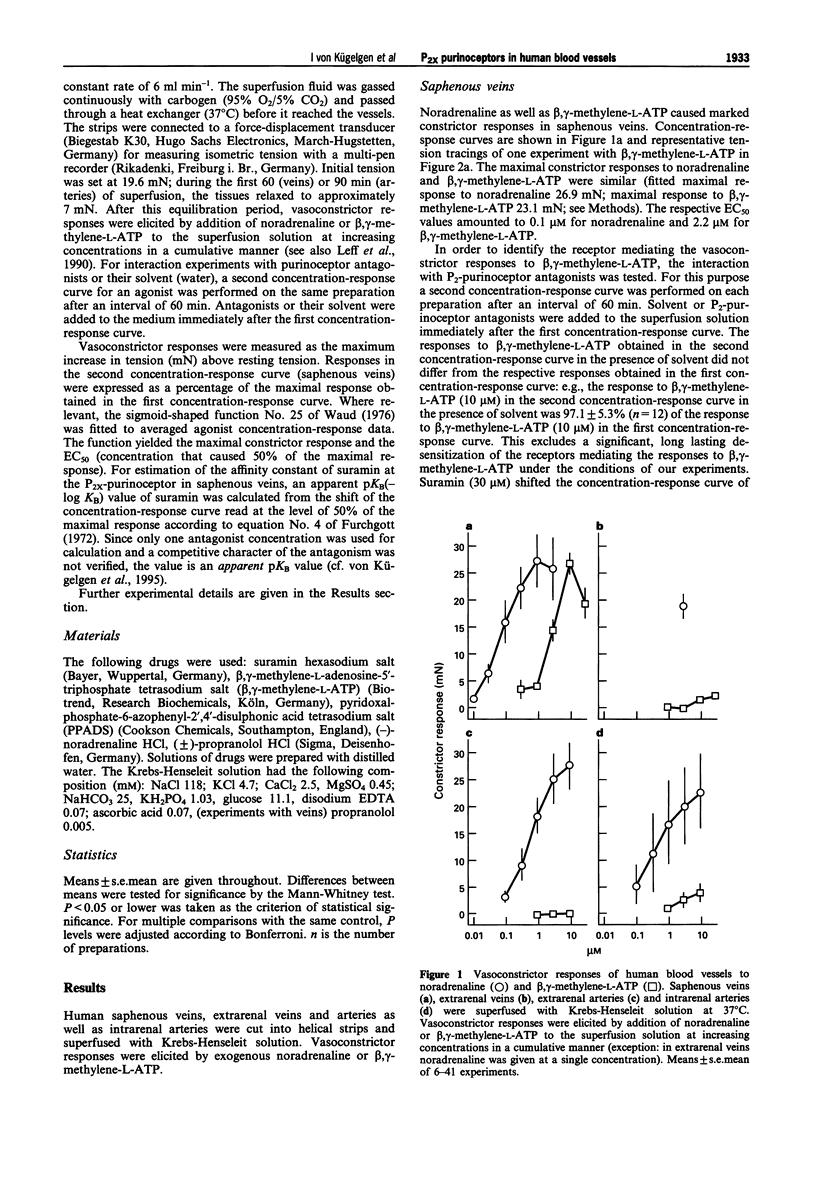
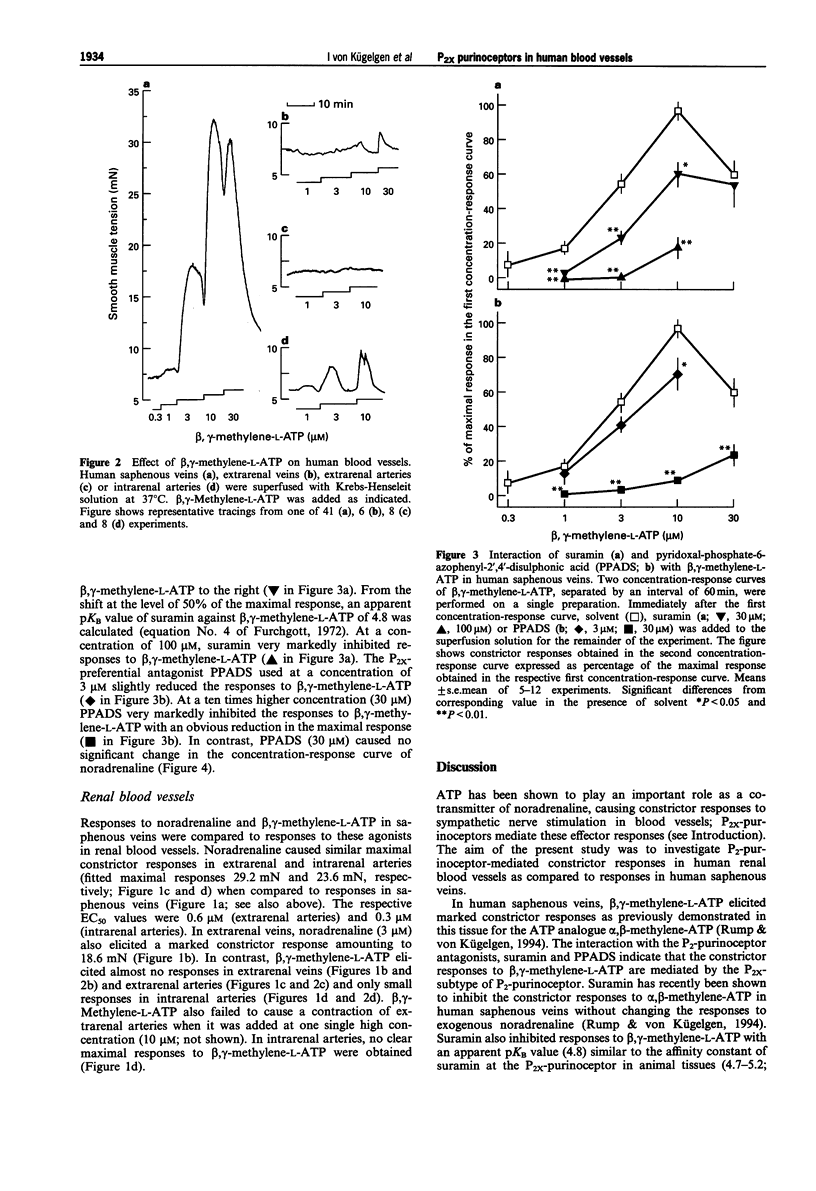
1. Strips of human saphenous veins and of human renal arteries and veins were superfused with Krebs-Henseleit solution at 37 degrees C. Constrictor responses were elicited by exogenous noradrenaline and the P2x-purinoceptor-selective agonist, beta, gamma-methylene-L-ATP. 2. In human saphenous veins, beta, gamma-methylene-L-ATP (0.3-30 microM; EC50 2.2 microM) induced marked constrictor responses. The maximal response to beta, gamma-methylene-L-ATP was similar to the maximal response to noradrenaline. The P2-purinoceptor antagonist suramin (30 microM) shifted the concentration-response curve of beta, gamma-methylene-L-ATP to the right (apparent pKB value 4.8); suramin (100 microM) markedly inhibited the responses to beta, gamma-methylene-L-ATP. The preferential P2x-purinoceptor antagonist, pyridoxal-phosphate-6-azophenyl-2',4'-disulphonic acid (PPADS; 3 microM) slightly reduced the response to beta, gamma-methylene-L-ATP. At a ten times higher concentration (30 microM), PPADS almost abolished the responses to beta, gamma-methylene-L-ATP. PPADS (30 microM), in contrast, caused no significant change in the concentration-response curve of noradrenaline. 3. In extrarenal and intrarenal arteries, EC50 values and maximal responses to noradrenaline were similar when compared with responses to noradrenaline in saphenous veins. Noradrenaline also constricted extrarenal veins. However, in contrast to the results obtained on saphenous veins, beta, gamma-methylene-L-ATP caused almost no constrictor responses in extrarenal veins and arteries and only moderate responses in intrarenal arteries. 4. The results demonstrate marked differences in responsiveness of human blood vessels to the selective P2x-purinoceptor agonist, beta, gamma-methylene-L-ATP, suggesting tissue differences in the occurrence or operation of P2x-purinoceptors in human vascular tissues. Moreover, the results indicate that PPADS blocks P2x-purinoceptors in human isolated blood vessels as previously demonstrated in animal blood vessels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbracchio M. P., Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Bohmann C., Rump L. C., Schaible U., von Kügelgen I. Alpha-adrenoceptor modulation of norepinephrine and ATP release in isolated kidneys of spontaneously hypertensive rats. Hypertension. 1995 Jun;25(6):1224–1231. doi: 10.1161/01.hyp.25.6.1224. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co-transmission. Arch Int Pharmacodyn Ther. 1990 Mar-Apr;304:7–33. [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardebo J. E., Kåhrström J., Owman C. P1- and P2-purine receptors in brain circulation. Eur J Pharmacol. 1987 Dec 15;144(3):343–352. doi: 10.1016/0014-2999(87)90387-6. [DOI] [PubMed] [Google Scholar]
- Hourani S. M., Loizou G. D., Cusack N. J. Pharmacological effects of L-AMP-PCP on ATP receptors in smooth muscle. Eur J Pharmacol. 1986 Nov 12;131(1):99–103. doi: 10.1016/0014-2999(86)90521-2. [DOI] [PubMed] [Google Scholar]
- Hourani S. M., Welford L. A., Cusack N. J. L-AMP-PCP, an ATP receptor agonist in guinea-pig bladder, is inactive on taenia coli. Eur J Pharmacol. 1985 Jan 22;108(2):197–200. doi: 10.1016/0014-2999(85)90726-5. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inscho E. W., Mitchell K. D., Navar L. G. Extracellular ATP in the regulation of renal microvascular function. FASEB J. 1994 Mar 1;8(3):319–328. doi: 10.1096/fasebj.8.3.8143938. [DOI] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Lambrecht G., Friebe T., Grimm U., Windscheif U., Bungardt E., Hildebrandt C., Bäumert H. G., Spatz-Kümbel G., Mutschler E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur J Pharmacol. 1992 Jul 7;217(2-3):217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- Leff P., Wood B. E., O'Connor S. E. Suramin is a slowly-equilibrating but competitive antagonist at P2x-receptors in the rabbit isolated ear artery. Br J Pharmacol. 1990 Nov;101(3):645–649. doi: 10.1111/j.1476-5381.1990.tb14134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. F., McCormack D. G., Evans T. W., Barnes P. J. Evidence for two P2-purinoceptor subtypes in human small pulmonary arteries. Br J Pharmacol. 1989 Nov;98(3):1014–1020. doi: 10.1111/j.1476-5381.1989.tb14633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. N., Thom S. A., Sever P. S. The effects of adenosine triphosphate (ATP) and related purines on human isolated subcutaneous and omental resistance arteries. Br J Pharmacol. 1991 Mar;102(3):645–650. doi: 10.1111/j.1476-5381.1991.tb12227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Pernow J., Svenberg T., Lundberg J. M. Actions of calcium antagonists on pre- and postjunctional effects of neuropeptide Y on human peripheral blood vessels in vitro. Eur J Pharmacol. 1987 Apr 14;136(2):207–218. doi: 10.1016/0014-2999(87)90712-6. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation. 1991 Jul;84(1):1–14. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- Rump L. C., Wilde K., Bohmann C., Schollmeyer P. Effects of the novel dopamine DA2-receptor agonist carmoxirole (EMD 45609) on noradrenergic and purinergic neurotransmission in rat isolated kidney. Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):300–308. doi: 10.1007/BF00168691. [DOI] [PubMed] [Google Scholar]
- Rump L. C., Wilde K., Schollmeyer P. Prostaglandin E2 inhibits noradrenaline release and purinergic pressor responses to renal nerve stimulation at 1 Hz in isolated kidneys of young spontaneously hypertensive rats. J Hypertens. 1990 Oct;8(10):897–908. doi: 10.1097/00004872-199010000-00003. [DOI] [PubMed] [Google Scholar]
- Rump L. C., von Kügelgen I. A study of ATP as a sympathetic cotransmitter in human saphenous vein. Br J Pharmacol. 1994 Jan;111(1):65–72. doi: 10.1111/j.1476-5381.1994.tb14024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. D., Malik K. U. Renal periarterial nerve stimulation-induced vasoconstriction at low frequencies is primarily due to release of a purinergic transmitter in the rat. J Pharmacol Exp Ther. 1989 Sep;250(3):764–771. [PubMed] [Google Scholar]
- Sehic E., Ruan Y., Malik K. U. Attenuation by alpha,beta-methylenadenosine-5'-triphosphate of periarterial nerve stimulation-induced renal vasoconstriction is not due to desensitization of purinergic receptors. J Pharmacol Exp Ther. 1994 Nov;271(2):983–992. [PubMed] [Google Scholar]
- Stones R. W., Beard R. W., Burnstock G. Pharmacology of the human ovarian vein: responses to putative neurotransmitters and endothelin-1. Br J Obstet Gynaecol. 1994 Aug;101(8):701–706. doi: 10.1111/j.1471-0528.1994.tb13188.x. [DOI] [PubMed] [Google Scholar]
- Windscheif U., Ralevic V., Bäumert H. G., Mutschler E., Lambrecht G., Burnstock G. Vasoconstrictor and vasodilator responses to various agonists in the rat perfused mesenteric arterial bed: selective inhibition by PPADS of contractions mediated via P2x-purinoceptors. Br J Pharmacol. 1994 Nov;113(3):1015–1021. doi: 10.1111/j.1476-5381.1994.tb17094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziganshin A. U., Hoyle C. H., Lambrecht G., Mutschler E., Bümert H. G., Burnstock G. Selective antagonism by PPADS at P2X-purinoceptors in rabbit isolated blood vessels. Br J Pharmacol. 1994 Mar;111(3):923–929. doi: 10.1111/j.1476-5381.1994.tb14827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kügelgen I., Bültmann R., Starke K. Effects of suramin and alpha, beta-methylene ATP indicate noradrenaline-ATP co-transmission in the response of the mouse vas deferens to single and low frequency pulses. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):760–763. doi: 10.1007/BF00169686. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Bültmann R., Starke K. Interaction of adenine nucleotides, UTP and suramin in mouse vas deferens: suramin-sensitive and suramin-insensitive components in the contractile effect of ATP. Naunyn Schmiedebergs Arch Pharmacol. 1990 Aug;342(2):198–205. doi: 10.1007/BF00166965. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol Sci. 1991 Sep;12(9):319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Stoffel D., Starke K. P2-purinoceptor-mediated inhibition of noradrenaline release in rat atria. Br J Pharmacol. 1995 May;115(2):247–254. doi: 10.1111/j.1476-5381.1995.tb15870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


