Abstract
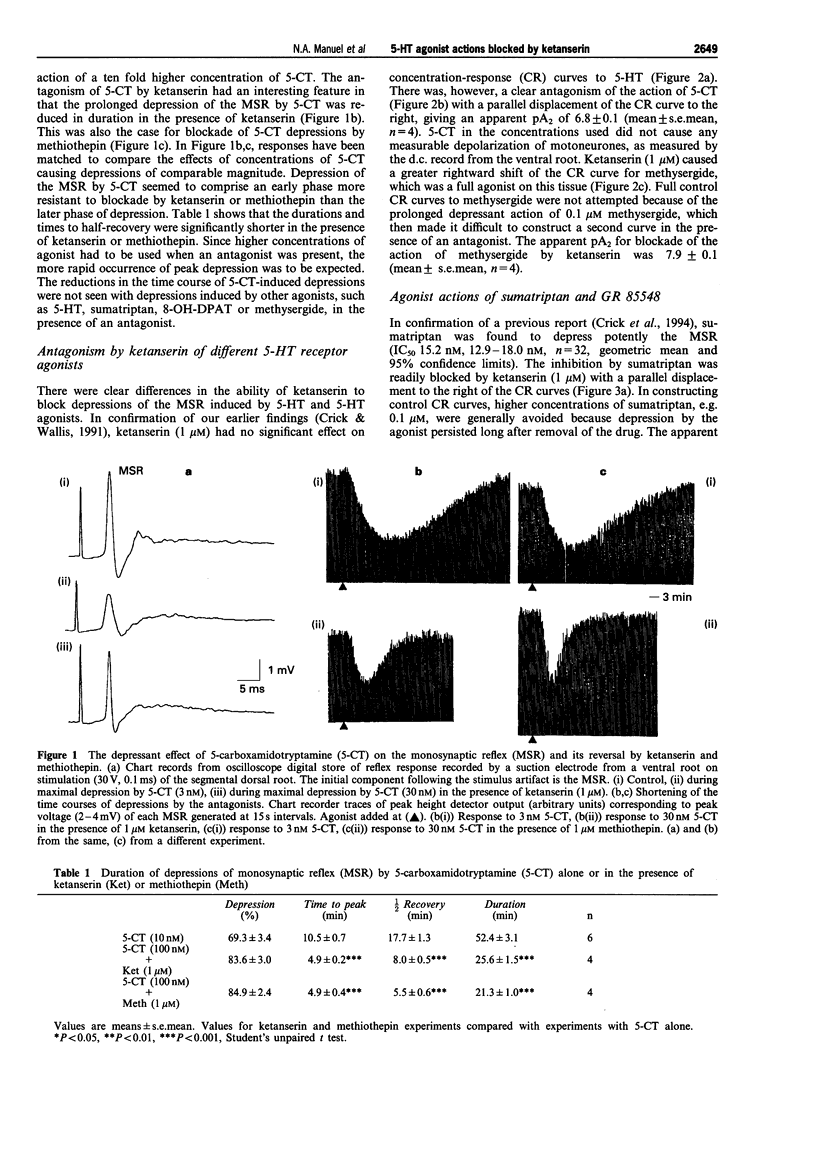
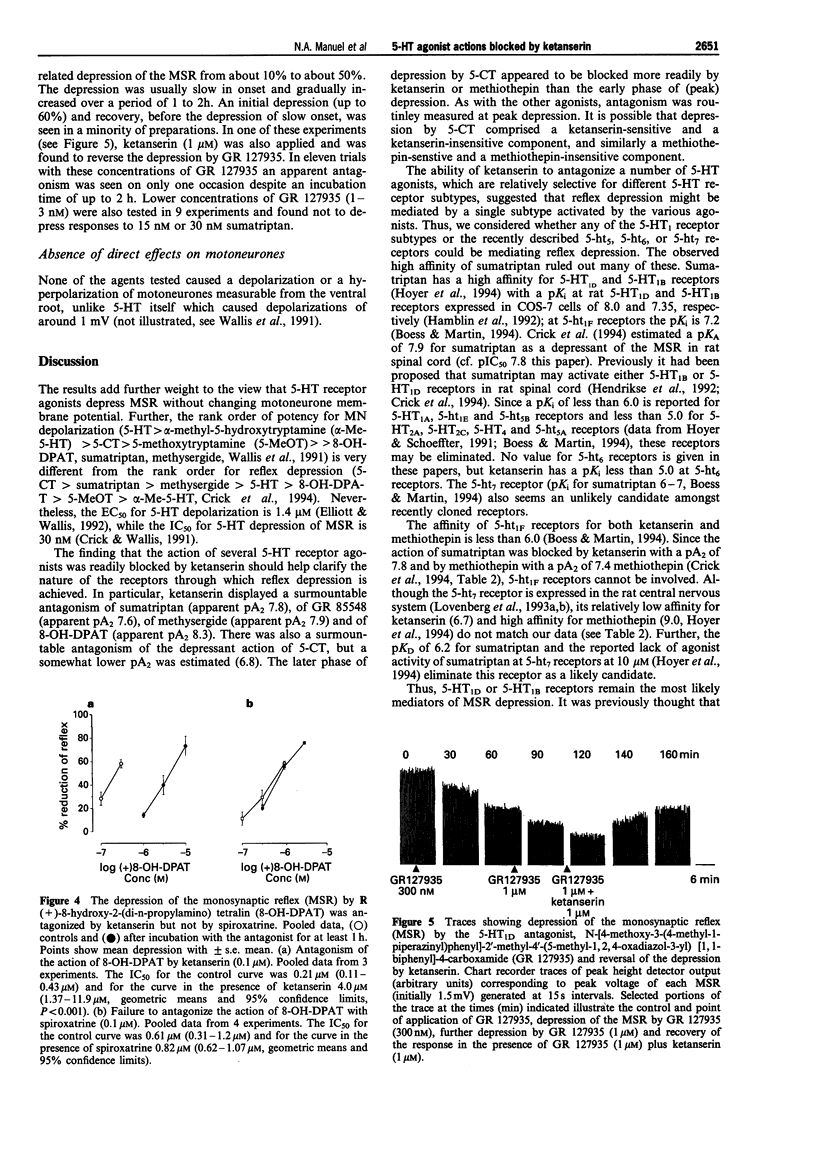
1. The monosynaptic reflex (MSR), recorded in vitro from the neonatal rat spinal cord, was depressed by 5-hydroxytryptamine (5-HT), 5-carboxamidotryptamine (5-CT), methysergide and R(+)-8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), and also by the selective 5-HT1D agonists, sumatriptan and N-methyl-3-(1-methyl-1-piperidinyl)-1H-indole-5-ethane sulphonamide (GR 85548). 2. Ketanserin (1 microM) and methiothepin (1 microM) reduced the duration of depressions elicited by 5-CT, but not those produced by 5-HT, sumatriptan, GR 85548, methysergide or 8-OH-DPAT. 3. The IC50 for MSR depression by 5-CT was 3.6, 2.1-6.2 nM (n = 4), by sumatriptan was 15.2, 12.9-18.0 nM (n = 32), by GR 85548 was 18.4, 11.7-29.1 nM (n = 12), by methysergide was 29.8, 10.2-87.1 nM (n = 4) and by 8-OH-DPAT was 0.21, 0.11-0.43 microM (n = 3) (geometric means and 95% confidence limits). 4. Ketanserin (0.1 or 1 microM) antagonized competitively responses to sumatriptan (apparent pA2 7.8 +/- 0.1, n = 5), GR 85548 (apparent pA2 7.6, unpaired data, n = 5), methysergide (apparent pA2 7.9 +/- 0.12, n = 4) and 8-OH-DPAT (apparent pA2 8.3 +/- 0.1, n = 3). Concentration-response curves to 5-CT showed a smaller, parallel shift to the right (apparent pA2 6.8 +/- 0.1, n = 4), but responses to 5-HT were unaffected by ketanserin (1 microM) (n = 4). 5. Methiothepin (1 microM) antagonized competitively responses to GR 85548 (apparent pA2 7.7, unpaired data, n = 5). 6. Mianserin (0.3 microM), a concentration sufficient to cause substantial block of 5-HT2C-mediated responses but have only a small effect on 5-HT1D-mediated actions, caused a small, non-parallel shift of the concentration-response curve to sumatriptan. 7. Depression of the MSR by sumatriptan was not blocked by (+/-)-cyanopindolol (0.1 microM), (+/-)-propranolol (0.5 or 1 microM) or spiroxatrine (0.1 microM), and depression of MSR by 8-OH-DPAT was not blocked by spiroxatrine (0.1 microM). (+/-)-Cyanopindolol (0.1 and 1 microM) itself induced a slow depression of the MSR. 8. The novel 5-HT1D antagonist, N-[4-methyl-1-piperazinyl) phenyl]2'-methyl-4'-(5-methyl-1,2,4-oxadiazol-3-yl) [1,1-biphenyl]-4-carboxamide (GR 127935, 30 nM to 1 microM) caused a concentration-related depression of the reflex (up to 50%) usually slow in onset. Neither with these concentrations nor with concentrations in the range 1-3 nM was there any unequivocal blockade of responses to sumatriptan. 9. It is concluded that sumatriptan, GR 85548, methysergide and 8-OH-DPAT depress the MSR in the neonate rat spinal cord via ketanserin-sensitive receptors, which have some similarities to 5-HT1D alpha receptors but which are not blocked by GR 127935. 5-HT released by tryptaminergic pathways may act via the same receptors to depress the MSR. 5-HT applied to the cord probably acts via a different, possibly novel 5-HT receptor to depress the MSR.
Full text
PDF

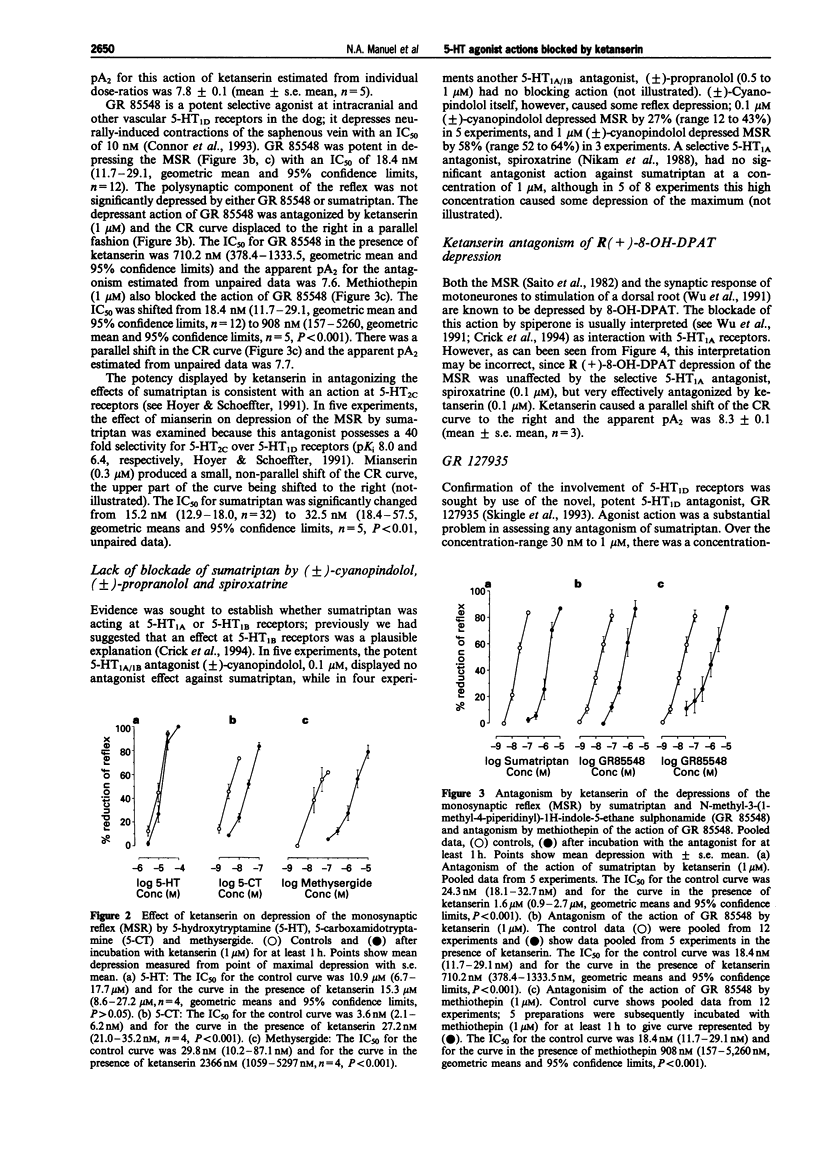



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach A. W., Unger L., Sprengel R., Mengod G., Palacios J., Seeburg P. H., Voigt M. M. Structure functional expression and spatial distribution of a cloned cDNA encoding a rat 5-HT1D-like receptor. J Recept Res. 1993;13(1-4):479–502. doi: 10.3109/10799899309073674. [DOI] [PubMed] [Google Scholar]
- Boess F. G., Martin I. L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994 Mar-Apr;33(3-4):275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Bruinvels A. T., Landwehrmeyer B., Gustafson E. L., Durkin M. M., Mengod G., Branchek T. A., Hoyer D., Palacios J. M. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994 Mar-Apr;33(3-4):367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Crick H., Manuel N. A., Wallis D. I. A novel 5-HT receptor or a combination of 5-HT receptor subtypes may mediate depression of a spinal monosynaptic reflex in vitro. Neuropharmacology. 1994 Jul;33(7):897–904. doi: 10.1016/0028-3908(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Crick H., Wallis D. I. Inhibition of reflex responses of neonate rat lumbar spinal cord by 5-hydroxytryptamine. Br J Pharmacol. 1991 Jul;103(3):1769–1775. doi: 10.1111/j.1476-5381.1991.tb09861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C., Stamford J. A. Evidence that 5-hydroxytryptamine release in rat dorsal raphé nucleus is controlled by 5-HT1A, 5-HT1B and 5-HT1D autoreceptors. Br J Pharmacol. 1995 Mar;114(6):1107–1109. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande S. B., Warnick J. E. Temperature-dependence of reflex transmission in the neonatal rat spinal cord, in vitro: influence on strychnine- and bicuculline-sensitive inhibition. Neuropharmacology. 1988 Oct;27(10):1033–1037. doi: 10.1016/0028-3908(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Elliott P., Wallis D. I. Serotonin and L-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience. 1992;47(3):533–544. doi: 10.1016/0306-4522(92)90163-v. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K., Titeler M. Detection and characterization of the serotonin 5-HT 1D receptor in rat and human brain. J Neurochem. 1988 May;50(5):1624–1631. doi: 10.1111/j.1471-4159.1988.tb03052.x. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994 Jun;46(2):157–203. [PubMed] [Google Scholar]
- Hoyer D., Middlemiss D. N. Species differences in the pharmacology of terminal 5-HT autoreceptors in mammalian brain. Trends Pharmacol Sci. 1989 Apr;10(4):130–132. doi: 10.1016/0165-6147(89)90159-4. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Schoeffter P. 5-HT receptors: subtypes and second messengers. J Recept Res. 1991;11(1-4):197–214. doi: 10.3109/10799899109066399. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., White S. R. Receptor subtypes mediating facilitation by serotonin of excitability of spinal motoneurons. Neuropharmacology. 1990 Sep;29(9):787–797. doi: 10.1016/0028-3908(90)90151-g. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Parsons A. A., Brown A. M. Human arterial constrictor serotonin receptors. Cardiovasc Res. 1993 Dec;27(12):2094–2103. doi: 10.1093/cvr/27.12.2094. [DOI] [PubMed] [Google Scholar]
- Larkman P. M., Kelly J. S. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. J Physiol. 1992 Oct;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg T. W., Baron B. M., de Lecea L., Miller J. D., Prosser R. A., Rea M. A., Foye P. E., Racke M., Slone A. L., Siegel B. W. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993 Sep;11(3):449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- Lovenberg T. W., Erlander M. G., Baron B. M., Racke M., Slone A. L., Siegel B. W., Craft C. M., Burns J. E., Danielson P. E., Sutcliffe J. G. Molecular cloning and functional expression of 5-HT1E-like rat and human 5-hydroxytryptamine receptor genes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2184–2188. doi: 10.1073/pnas.90.6.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y., Nakano S., Yasuda H. Postnatal transient expression of long-lasting descending inhibitory effect on spinal reflexes in the rat. Neurosci Res. 1987 Apr;4(4):268–278. doi: 10.1016/0168-0102(87)90043-5. [DOI] [PubMed] [Google Scholar]
- Nikam S. S., Martin A. R., Nelson D. L. Serotonergic properties of spiroxatrine enantiomers. J Med Chem. 1988 Oct;31(10):1965–1968. doi: 10.1021/jm00118a017. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Electrophysiology of mammalian spinal cord in vitro. Nature. 1974 Dec 20;252(5485):733–734. doi: 10.1038/252733a0. [DOI] [PubMed] [Google Scholar]
- Parsons A. A., Whalley E. T., Feniuk W., Connor H. E., Humphrey P. P. 5-HT1-like receptors mediate 5-hydroxytryptamine-induced contraction of human isolated basilar artery. Br J Pharmacol. 1989 Feb;96(2):434–440. doi: 10.1111/j.1476-5381.1989.tb11835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels P. J., Colpaert F. C. The 5-HT1D receptor antagonist GR 127,935 is an agonist at cloned human 5-HT1D alpha receptor sites. Neuropharmacology. 1995 Feb;34(2):235–237. doi: 10.1016/0028-3908(95)00007-s. [DOI] [PubMed] [Google Scholar]
- Saito K., Ito S., Kitazawa T., Ohga A. Selective inhibition by methysergide of the monosynaptic reflex discharge in the isolated spinal cord of the newborn rat. Brain Res. 1982 Nov 11;251(1):117–125. doi: 10.1016/0006-8993(82)91279-3. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., Connell L. A., Kvaltinova Z. Further studies on the action of 5-hydroxytryptamine on lumbar motoneurones in the rat isolated spinal cord. Naunyn Schmiedebergs Arch Pharmacol. 1991 Apr;343(4):344–352. doi: 10.1007/BF00179038. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., Wu J., Wang X. C. Is 5-hydroxytryptamine mediating descending inhibition in the neonatal rat spinal cord through different receptor subtypes? Eur J Pharmacol. 1993 Dec 21;250(3):371–377. doi: 10.1016/0014-2999(93)90023-b. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., Wu J., Wang X. Descending inhibition in the neonate rat spinal cord is mediated by 5-hydroxytryptamine. Neuropharmacology. 1993 Jan;32(1):73–83. doi: 10.1016/0028-3908(93)90132-m. [DOI] [PubMed] [Google Scholar]
- Wang M. Y., Dun N. J. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990 Nov;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshank R. L., Zgombick J. M., Macchi M. J., Branchek T. A., Hartig P. R. Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1D alpha and 5-HT1D beta. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3630–3634. doi: 10.1073/pnas.89.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson L. O., Middlemiss D. N. Metitepine distinguishes two receptors mediating inhibition of [3H]-5-hydroxytryptamine release in guinea pig hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1992 Jun;345(6):696–699. doi: 10.1007/BF00164585. [DOI] [PubMed] [Google Scholar]
- Wu S. Y., Wang M. Y., Dun N. J. Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res. 1991 Jul 19;554(1-2):111–121. doi: 10.1016/0006-8993(91)90178-x. [DOI] [PubMed] [Google Scholar]
- Yomono H. S., Suzuki H., Yoshioka K. Serotonergic fibers induce a long-lasting inhibition of monosynaptic reflex in the neonatal rat spinal cord. Neuroscience. 1992;47(3):521–531. doi: 10.1016/0306-4522(92)90162-u. [DOI] [PubMed] [Google Scholar]
- van Wijngaarden I., Tulp M. T., Soudijn W. The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990 Jun 12;188(6):301–312. doi: 10.1016/0922-4106(90)90190-9. [DOI] [PubMed] [Google Scholar]


