Abstract
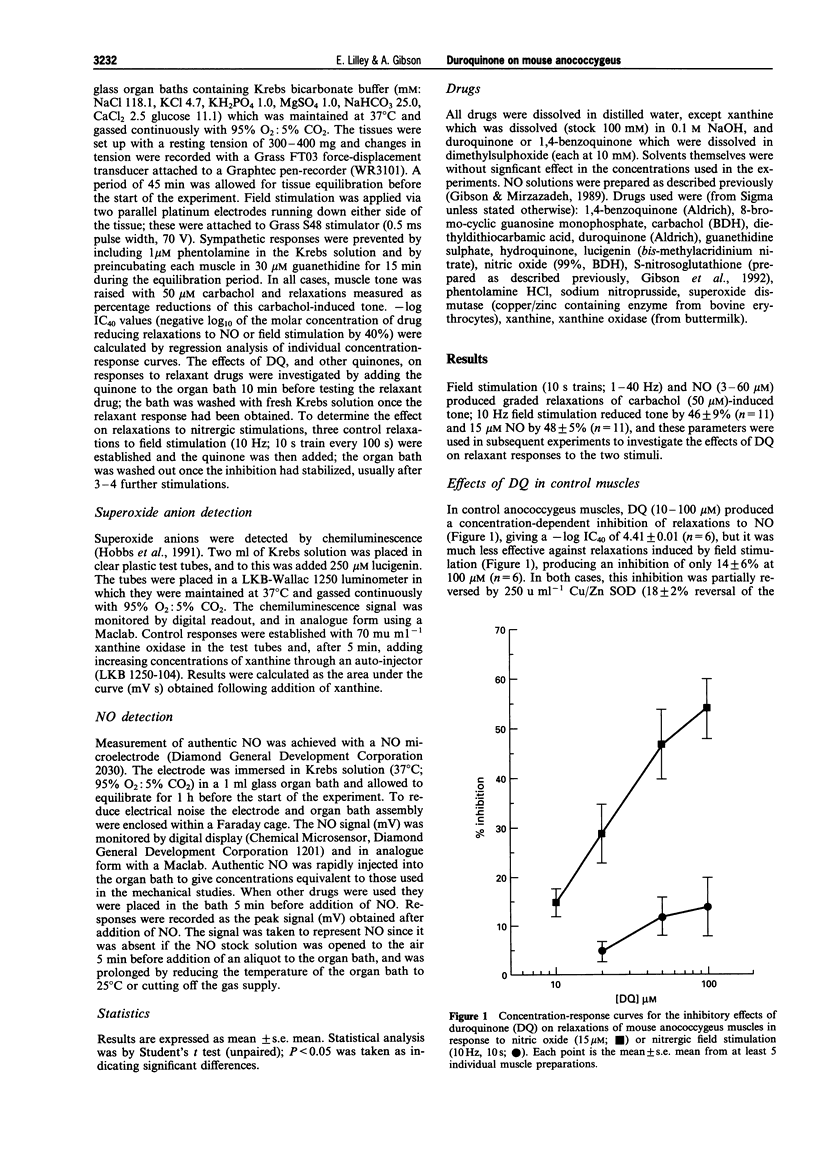
1. The role of copper/zinc superoxide dismutase (Cu/Zn SOD) in protection of nitrergic neurotransmission in the mouse anococcygeus was investigated by use of duroquinone (DQ), which generates superoxide anions within tissues via reduction by flavoprotein enzymes. 2. In control anococcygeus muscles, DQ (10-100 microM) produced concentration-related inhibition (-log IC40 = 4.41) of relaxations to exogenous nitric oxide (NO; 15 microM). Nitrergic relaxations induced by field stimulation (10 Hz; 10 s train) were much less affected, 100 microM DQ reducing nitrergic relaxations by only 14 +/- 6%. 3. Following incubation with the Cu/Zn SOD inhibitor, diethyldithiocarbamate (DETCA; 3 mM; 45 min incubation; 10 min washout), the inhibitory effects of DQ on relaxations to NO were potentiated (-log IC40 = 5.22), and clear, concentration-related inhibitions of nitrergic relaxations were now observed (-log IC40 = 4.54). In both cases, these inhibitions were partially reversed by Cu/Zn SOD (250 u ml-1). In DETCA-treated tissues, DQ (100 microM) also reduced relaxations to sodium nitroprusside (1 microM) and S-nitroso-glutathione (30 microM), but potentiated those to 8-Br-cyclic GMP (100 microM). 4. Neither hydroquinone (HQ: 100 microM) nor 1,4-benzoquinone (BQ: 100 microM), both of which reduced responses to exogenous NO, inhibited relaxations induced by field stimulation in DETCA-treated tissues. Indeed, when added during DQ-induced inhibition of nitrergic relaxations, both HQ and BQ produced partial reversal of the block. 5. DQ had no effect on the detection of superoxide anions estimated via the xanthine:xanthine oxidase chemiluminescence assay, or of authentic NO as measured by a chemical microsensor. However, the detection of both superoxide anions and NO in these assays was inhibited by inclusion of either HQ or BQ. 6. The results support the proposal that nitrergic transmission in the peripheral nervous system is protected by Cu/Zn SOD activity in the region of the neuroeffector junction, and this may explain the lack of effect of superoxide anion generating drugs such as DQ. Such an explanation does not hold for either HQ or BQ, which appear to be acting directly as free radical scavengers in these experiments.
Full text
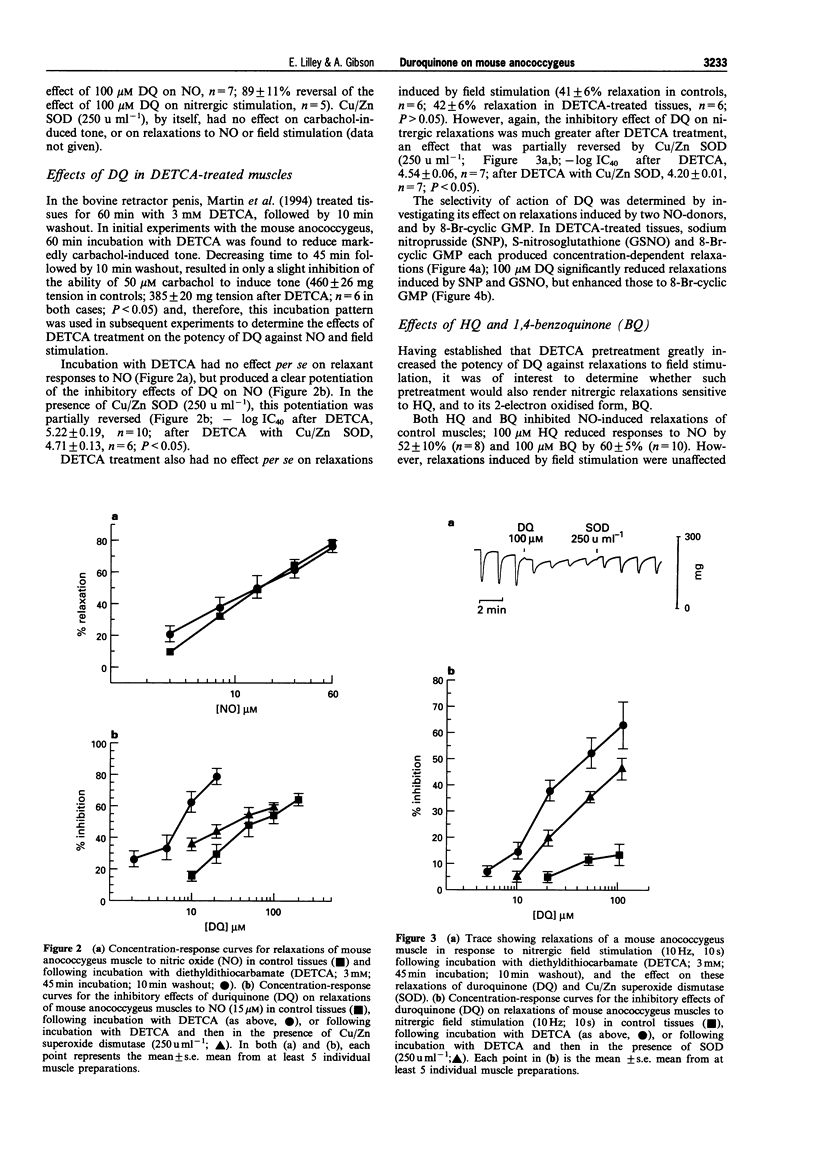
PDF
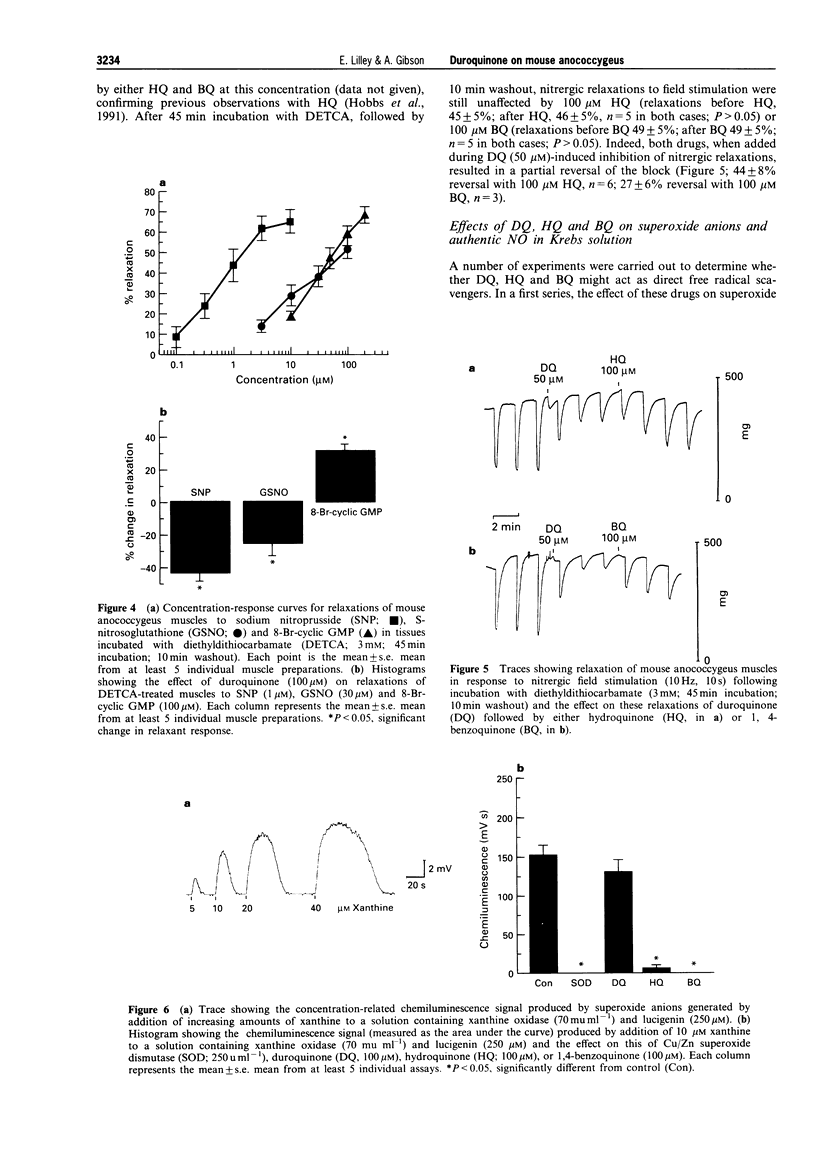

Selected References
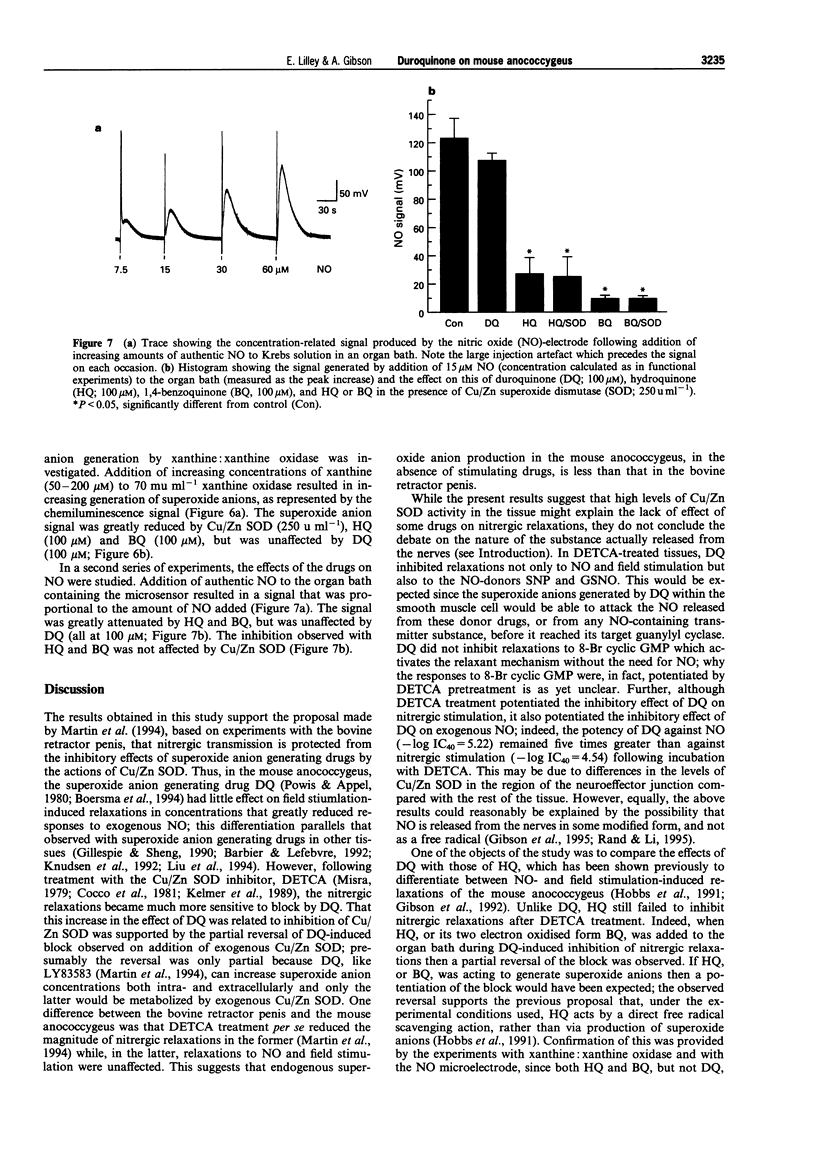
These references are in PubMed. This may not be the complete list of references from this article.
- Barbier A. J., Lefebvre R. A. Effect of LY 83583 on relaxation induced by non-adrenergic non-cholinergic nerve stimulation and exogenous nitric oxide in the rat gastric fundus. Eur J Pharmacol. 1992 Aug 25;219(2):331–334. doi: 10.1016/0014-2999(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Boersma M. G., Balvers W. G., Boeren S., Vervoort J., Rietjens I. M. NADPH-cytochrome reductase catalysed redox cycling of 1,4-benzoquinone; hampered at physiological conditions, initiated at increased pH values. Biochem Pharmacol. 1994 Jun 1;47(11):1949–1955. doi: 10.1016/0006-2952(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Cocco D., Calabrese L., Rigo A., Argese E., Rotilio G. Re-examination of the reaction of diethyldithiocarbamate with the copper of superoxide dismutase. J Biol Chem. 1981 Sep 10;256(17):8983–8986. [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Brave S. R., McFadzean I., Tucker J. F., Wayman C. The nitrergic transmitter of the anococcygeus--NO or not? Arch Int Pharmacodyn Ther. 1995 Jan-Feb;329(1):39–51. [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs. Br J Pharmacol. 1989 Mar;96(3):637–644. doi: 10.1111/j.1476-5381.1989.tb11863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscles. Br J Pharmacol. 1990 Jan;99(1):194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Tucker J. F., Gibson A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br J Pharmacol. 1991 Nov;104(3):645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner M. J., Bagnell R., Hale B., Alexander N. M. Inactivation of intracellular copper-zinc superoxide dismutase by copper chelating agents without glutathione depletion and methemoglobin formation. Free Radic Biol Med. 1989;6(4):355–360. doi: 10.1016/0891-5849(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Knudsen M. A., Svane D., Tøttrup A. Action profiles of nitric oxide, S-nitroso-L-cysteine, SNP, and NANC responses in opossum lower esophageal sphincter. Am J Physiol. 1992 May;262(5 Pt 1):G840–G846. doi: 10.1152/ajpgi.1992.262.5.G840. [DOI] [PubMed] [Google Scholar]
- Lau S. S., Hill B. A., Highet R. J., Monks T. J. Sequential oxidation and glutathione addition to 1,4-benzoquinone: correlation of toxicity with increased glutathione substitution. Mol Pharmacol. 1988 Dec;34(6):829–836. [PubMed] [Google Scholar]
- Liu X., Gillespie J. S., Martin W. Non-adrenergic, non-cholinergic relaxation of the bovine retractor penis muscle: role of S-nitrosothiols. Br J Pharmacol. 1994 Apr;111(4):1287–1295. doi: 10.1111/j.1476-5381.1994.tb14885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., McAllister K. H., Paisley K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994 Nov;33(11):1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Misra H. P. Reaction of copper-zinc superoxide dismutase with diethyldithiocarbamate. J Biol Chem. 1979 Nov 25;254(22):11623–11628. [PubMed] [Google Scholar]
- Powis G., Appel P. L. Relationship of the single-electron reduction potential of quinones to their reduction by flavoproteins. Biochem Pharmacol. 1980 Oct 1;29(19):2567–2572. doi: 10.1016/0006-2952(80)90068-4. [DOI] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Differential effects of hydroxocobalamin on relaxations induced by nitrosothiols in rat aorta and anococcygeus muscle. Eur J Pharmacol. 1993 Sep 14;241(2-3):249–254. doi: 10.1016/0014-2999(93)90210-9. [DOI] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Rao D. N., Takahashi N., Mason R. P. Characterization of a glutathione conjugate of the 1,4-benzosemiquinone-free radical formed in rat hepatocytes. J Biol Chem. 1988 Dec 5;263(34):17981–17986. [PubMed] [Google Scholar]
- Wood J., Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994 Nov;33(11):1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]


