Abstract
1. Previous studies have shown that endothelium-dependent relaxation in the aorta of spontaneously diabetic bio bred rats (BB) is impaired. 2. We have investigated noradrenaline (NA) contractility, endothelium-dependent acetylcholine (ACh) and bradykinin (BK) relaxation, and endothelium-independent sodium nitroprusside (SNP) relaxation in mesenteric resistance arteries of recent onset BB rats and established insulin treated BB rats, compared to their age-matched non diabetic controls. 3. There was no significant difference in the maximum contractile response or sensitivity to noradrenaline in either of the diabetic groups compared to their age-matched controls. 4. Incubation with the nitric oxide synthetase inhibitor NG-nitro-L-arginine (L-NOARG) resulted in a significant increase in maximum contractile response to noradrenaline in the recent onset age-matched control group (P < 0.05). Analysis of the whole dose-response curve (using ANOVA for repeated measures with paired t test) showed a significant left-ward shift following the addition of L-NOARG (P < 0.001). A similar but less marked shift (P < 0.01) was evident in vessels from recent onset diabetics. An overall shift in both sensitivity and maximum response was also evident in the age-matched non diabetic controls of the insulin-treated group (P < 0.05). However, by contrast, there was no significant change in sensitivity in the insulin-treated diabetic rats. 5. ACh-induced endothelium-dependent relaxation was significantly impaired in the recent onset diabetic rats compared to their age-matched controls (47 +/- 11% versus 92 +/- 2%, P < 0.05, n = 6), and in the insulin treated diabetic rats (34 +/- 5% versus 75 +/- 6%, P < 0.05, n = 6). The relaxation responses to BK also were significantly impaired in the diabetic rats compared to their age-matched controls (recent onset: 20 +/- 3% versus 72 +/- 7%, P < 0.05, n = 6; insulin treated: 12 +/- 9% versus 68 +/- 7%, P < 0.05, n = 7). 6. Incubation with either the nitric oxide synthetase substrate, U-arginine, or the free radical scavenging enzyme superoxide dismutase (150 mu ml-1) failed to improve the attenuated response of acetylcholine-induced relaxation in the diabetic vessels. 7. Endothelium-dependent relaxation mediated by ACh and BK was significantly attenuated in both the diabetic and control vessels after incubation with L-NOARG. 8. Pretreatment with a cyclo-oxygenase inhibitor, indomethacin, significantly enhanced the relaxation to ACh in both the recent onset and insulin treated diabetic rats (42 +/- 10%, n = 7 versus 64 +/- 7%, n = 7, P < 0.05, and 40 +/- 5%, n = 7 versus 65 +/- 9%, n = 6, P < 0.05). 9. Following endothelium removal, there was a marked impairment in endothelium-dependent relaxation responses to ACh and BK in both the diabetic and control vessels. 10. Incubation with the thromboxane A2 receptor antagonist SQ29548, did not significantly improve the ACh endothelium-dependent relaxation response in the diabetic vessels. 11. Endothelium-independent relaxation to sodium nitroprusside was significantly impaired in the first group of diabetic vessels studied; however, subsequent studies showed no impairment of the sodium nitroprusside response in the diabetic vessels. 12. In conclusion, the ability of the endothelium to regulate vascular contractility is reduced in recent onset diabetic vessels, and significantly impaired in established insulin treated diabetics. Relaxation to the endothelium-dependent vasodilators ACh and BK was impaired in both the recent onset and the established insulin treated diabetics, and the ACh response was significantly improved following pretreatment with indomethacin, suggesting a role for a cyclo-oxygenase-derived vasoconstrictor. Preliminary studies with a thromboxane A2, receptor antagonist, SQ29548 did not significantly improve the impaired relaxation to ACh, indicating that the vasoconstrictor prostanoid is not thromboxane A2.
Full text
PDF


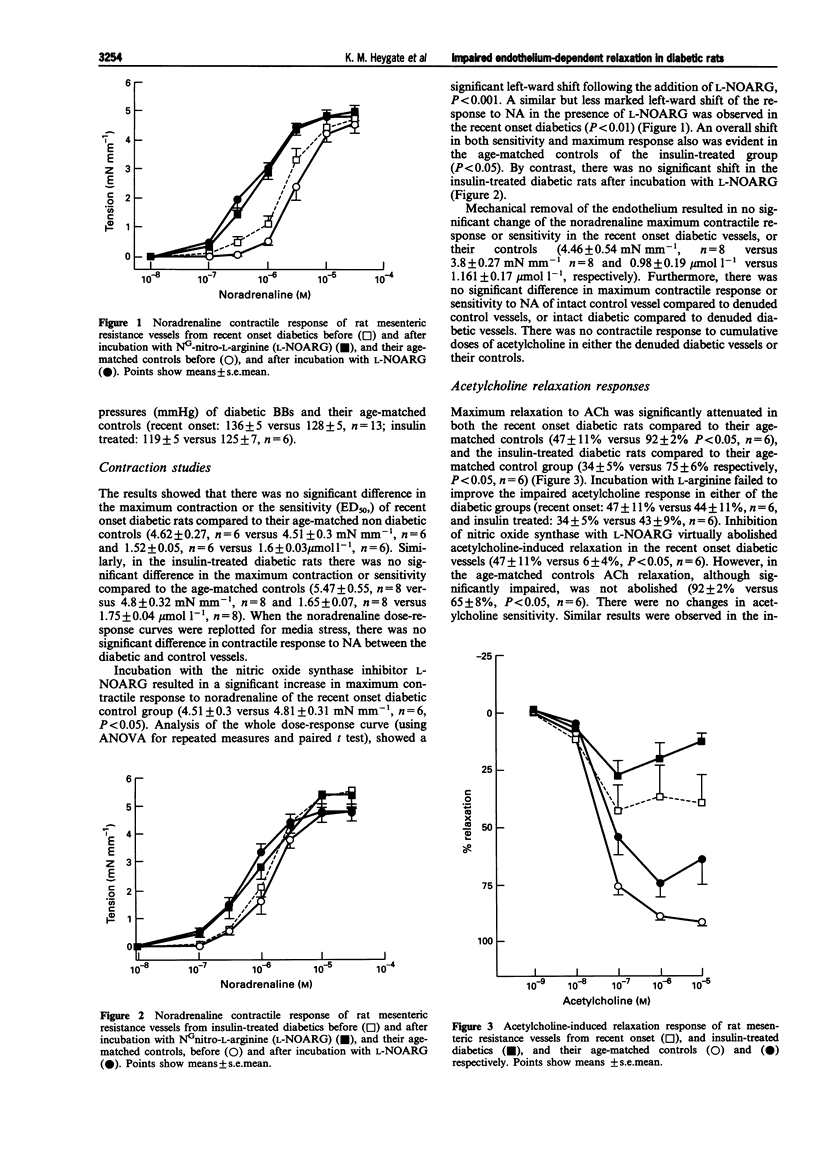
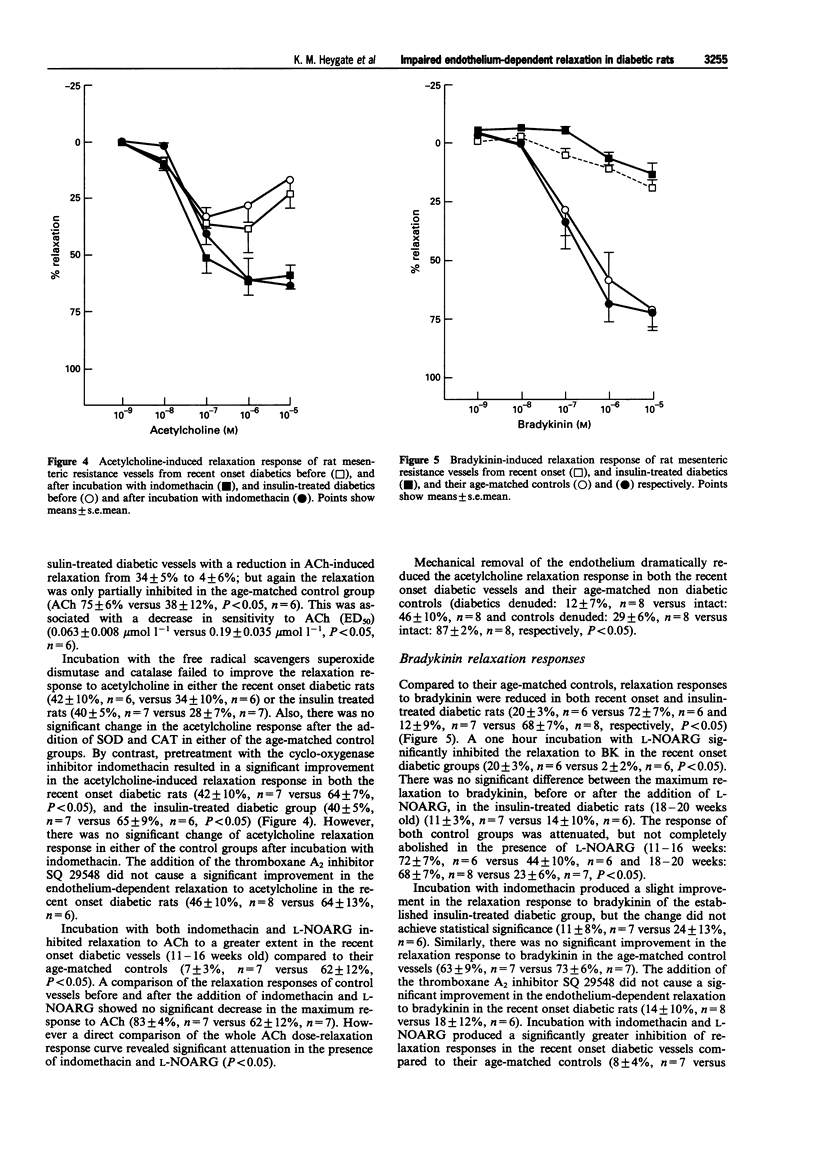




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. A., Watt P. A., Thurston H. Endothelium-dependent modulation of resistance vessel contraction: studies with NG-nitro-L-arginine methyl ester and NG-nitro-L-arginine. Br J Pharmacol. 1992 Oct;107(2):616–621. doi: 10.1111/j.1476-5381.1992.tb12792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. A., Watt P. A., Thurston H. Impaired endothelium-dependent relaxation in two-kidney, one clip Goldblatt hypertension: effect of vasoconstrictor prostanoids. J Hypertens Suppl. 1993 Dec;11(5):S134–S135. [PubMed] [Google Scholar]
- Buñag R. D. Validation in awake rats of a tail-cuff method for measuring systolic pressure. J Appl Physiol. 1973 Feb;34(2):279–282. doi: 10.1152/jappl.1973.34.2.279. [DOI] [PubMed] [Google Scholar]
- Calver A., Collier J., Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992 Dec;90(6):2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christlieb A. R., Janka H. U., Kraus B., Gleason R. E., Icasas-Cabral E. A., Aiello L. M., Cabral B. V., Solano A. Vascular reactivity to angiotensin II and to norepinephrine in diabetic subjects. Diabetes. 1976 Apr;25(4):268–274. doi: 10.2337/diab.25.4.268. [DOI] [PubMed] [Google Scholar]
- Cooper M. E., Rumble J., Komers R., Du H. C., Jandeleit K., Chou S. T. Diabetes-associated mesenteric vascular hypertrophy is attenuated by angiotensin-converting enzyme inhibition. Diabetes. 1994 Oct;43(10):1221–1228. doi: 10.2337/diab.43.10.1221. [DOI] [PubMed] [Google Scholar]
- Ditzel J., Junker K. Abnormal glomerular filtration rate, renal plasma flow, and renal protein excretion in recent and short-term diabetics. Br Med J. 1972 Apr 1;2(5804):13–19. doi: 10.1136/bmj.2.5804.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante W., Sen A. K., Sunahara F. A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988 Jun;94(2):463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. G., Cockcroft J. R., Groop P. H., Viberti G. C., Ritter J. M. Inhibition of nitric oxide synthesis in forearm vasculature of insulin-dependent diabetic patients: blunted vasoconstriction in patients with microalbuminuria. Clin Sci (Lond) 1993 Dec;85(6):687–693. doi: 10.1042/cs0850687. [DOI] [PubMed] [Google Scholar]
- Friedman J. J. Vascular sensitivity and reactivity to norepinephrine in diabetes mellitus. Am J Physiol. 1989 Apr;256(4 Pt 2):H1134–H1138. doi: 10.1152/ajpheart.1989.256.4.H1134. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garcia M. J., McNamara P. M., Gordon T., Kannel W. B. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974 Feb;23(2):105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Ha H., Dunham E. W. Limited capacity for renal vasodilatation in anesthetized diabetic rats. Am J Physiol. 1987 Oct;253(4 Pt 2):H845–H855. doi: 10.1152/ajpheart.1987.253.4.H845. [DOI] [PubMed] [Google Scholar]
- Halkin A., Benjamin N., Doktor H. S., Todd S. D., Viberti G., Ritter J. M. Vascular responsiveness and cation exchange in insulin-dependent diabetes. Clin Sci (Lond) 1991 Aug;81(2):223–232. doi: 10.1042/cs0810223. [DOI] [PubMed] [Google Scholar]
- Harris K. H., MacLeod K. M. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur J Pharmacol. 1988 Aug 9;153(1):55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- Head R. J., Longhurst P. A., Panek R. L., Stitzel R. E. A contrasting effect of the diabetic state upon the contractile responses of aortic preparations from the rat and rabbit. Br J Pharmacol. 1987 Jun;91(2):275–286. doi: 10.1111/j.1476-5381.1987.tb10282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben A. J., Schaper N. C., Slaaf D. W., Tangelder G. J., Nieuwenhuijzen Kruseman A. C. Skin blood cell flux in insulin-dependent diabetic subjects in relation to retinopathy or incipient nephropathy. Eur J Clin Invest. 1992 Jan;22(1):67–72. doi: 10.1111/j.1365-2362.1992.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Jennings P. E., Jones A. F., Florkowski C. M., Lunec J., Barnett A. H. Increased diene conjugates in diabetic subjects with microangiopathy. Diabet Med. 1987 Sep-Oct;4(5):452–456. doi: 10.1111/j.1464-5491.1987.tb00908.x. [DOI] [PubMed] [Google Scholar]
- Johnstone M. T., Creager S. J., Scales K. M., Cusco J. A., Lee B. K., Creager M. A. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993 Dec;88(6):2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989 Jun;97(2):614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohner E. M., Hamilton A. M., Saunders S. J., Sutcliffe B. A., Bulpitt C. J. The retinal blood flow in diabetes. Diabetologia. 1975 Feb;11(1):27–33. doi: 10.1007/BF00422814. [DOI] [PubMed] [Google Scholar]
- Legan E. Effects of streptozotocin-induced hyperglycemia on agonist-stimulated phosphatidylinositol turnover in rat aorta. Life Sci. 1989;45(5):371–378. doi: 10.1016/0024-3205(89)90622-x. [DOI] [PubMed] [Google Scholar]
- Ljusegren M. E., Axelsson K. L., Ahlner J., Karlsson J. O., Andersson R. G., Magnusson B. R., Friedman R. L. Effects of pertussis toxin on vasodilation and cyclic GMP in bovine mesenteric arteries and demonstration of a 40 kD soluble protein ribosylation substrate for pertussis toxin. Life Sci. 1990;46(8):543–552. doi: 10.1016/0024-3205(90)90121-7. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Boulanger C. M., Dohi Y., Yang Z. H. Endothelium-derived contracting factors. Hypertension. 1992 Feb;19(2):117–130. doi: 10.1161/01.hyp.19.2.117. [DOI] [PubMed] [Google Scholar]
- MacLeod K. M., McNeill J. H. The influence of chronic experimental diabetes on contractile responses of rat isolated blood vessels. Can J Physiol Pharmacol. 1985 Jan;63(1):52–57. doi: 10.1139/y85-009. [DOI] [PubMed] [Google Scholar]
- Marliss E. B., Nakhooda A. F., Poussier P., Sima A. A. The diabetic syndrome of the 'BB' Wistar rat: possible relevance to type 1 (insulin-dependent) diabetes in man. Diabetologia. 1982 Apr;22(4):225–232. doi: 10.1007/BF00281296. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G., Simmons L. K., Sharpe G. M. Mechanism of impaired responses of cerebral arterioles during diabetes mellitus. Am J Physiol. 1991 Feb;260(2 Pt 2):H319–H326. doi: 10.1152/ajpheart.1991.260.2.H319. [DOI] [PubMed] [Google Scholar]
- McNally P. G., Lawrence I. G., Watt P. A., Hillier C., Burden A. C., Thurston H. The effect of insulin on the vascular reactivity of isolated resistance arteries taken from healthy volunteers. Diabetologia. 1995 Apr;38(4):467–473. doi: 10.1007/BF00410285. [DOI] [PubMed] [Google Scholar]
- McNally P. G., Watt P. A., Rimmer T., Burden A. C., Hearnshaw J. R., Thurston H. Impaired contraction and endothelium-dependent relaxation in isolated resistance vessels from patients with insulin-dependent diabetes mellitus. Clin Sci (Lond) 1994 Jul;87(1):31–36. doi: 10.1042/cs0870031. [DOI] [PubMed] [Google Scholar]
- Meraji S., Jayakody L., Senaratne M. P., Thomson A. B., Kappagoda T. Endothelium-dependent relaxation in aorta of BB rat. Diabetes. 1987 Aug;36(8):978–981. doi: 10.2337/diab.36.8.978. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. A., Bohlen H. G., Miller B. G., Evan A. P. Cellular and vessel wall morphology of cerebral cortical arterioles after short-term diabetes in adult rats. Blood Vessels. 1985;22(6):265–277. doi: 10.1159/000158613. [DOI] [PubMed] [Google Scholar]
- Mordes J. P., Desemone J., Rossini A. A. The BB rat. Diabetes Metab Rev. 1987 Jul;3(3):725–750. doi: 10.1002/dmr.5610030307. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Mechanical properties of vascular smooth muscle cells in situ. Nature. 1976 Apr 15;260(5552):617–619. doi: 10.1038/260617a0. [DOI] [PubMed] [Google Scholar]
- Nakhooda A. F., Like A. A., Chappel C. I., Murray F. T., Marliss E. B. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes. 1977 Feb;26(2):100–112. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- Osol G., Cipolla M., Knutson S. A new method for mechanically denuding the endothelium of small (50-150 microns) arteries with a human hair. Blood Vessels. 1989;26(5):320–324. doi: 10.1159/000158781. [DOI] [PubMed] [Google Scholar]
- Owen M. P., Carrier G. O. Calcium dependence of norepinephrine-induced vascular contraction in experimental diabetes. J Pharmacol Exp Ther. 1980 Feb;212(2):253–258. [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Fry D. L. The elastic symmetry of arterial segments in dogs. Circ Res. 1969 Jan;24(1):1–8. doi: 10.1161/01.res.24.1.1. [DOI] [PubMed] [Google Scholar]
- Pfaffman M. A., Ball C. R., Darby A., Hilman R. Insulin reversal of diabetes-induced inhibition of vascular contractility in the rat. Am J Physiol. 1982 Apr;242(4):H490–H495. doi: 10.1152/ajpheart.1982.242.4.H490. [DOI] [PubMed] [Google Scholar]
- Pieper G. M., Gross G. J. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1988 Oct;255(4 Pt 2):H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- Porta M., La Selva M., Molinatti P., Molinatti G. M. Endothelial cell function in diabetic microangiopathy. Diabetologia. 1987 Aug;30(8):601–609. doi: 10.1007/BF00277315. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Sandeman D. D., Shore A. C., Tooke J. E. Relation of skin capillary pressure in patients with insulin-dependent diabetes mellitus to complications and metabolic control. N Engl J Med. 1992 Sep 10;327(11):760–764. doi: 10.1056/NEJM199209103271103. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Muramatsu M., Kakegawa Y., Asano H., Toki Y., Miyazaki Y., Okumura K., Hashimoto H., Ito T. Role of prostaglandin H2 as an endothelium-derived contracting factor in diabetic state. Diabetes. 1993 Sep;42(9):1246–1252. doi: 10.2337/diab.42.9.1246. [DOI] [PubMed] [Google Scholar]
- Steel M., Nolan C., Nankervis A., Kiers L., Kilpatrick C., Lichtenstein M., O'Dea K., Larkins R. Forearm arterial vascular responsiveness in insulin-dependent diabetic subjects. Diabetes Res Clin Pract. 1993 Aug-Sep;21(2-3):127–136. doi: 10.1016/0168-8227(93)90060-i. [DOI] [PubMed] [Google Scholar]
- Taylor P. D., Graves J. E., Poston L. Selective impairment of acetylcholine-mediated endothelium-dependent relaxation in isolated resistance arteries of the streptozotocin-induced diabetic rat. Clin Sci (Lond) 1995 May;88(5):519–524. doi: 10.1042/cs0880519. [DOI] [PubMed] [Google Scholar]
- Taylor P. D., McCarthy A. L., Thomas C. R., Poston L. Endothelium-dependent relaxation and noradrenaline sensitivity in mesenteric resistance arteries of streptozotocin-induced diabetic rats. Br J Pharmacol. 1992 Oct;107(2):393–399. doi: 10.1111/j.1476-5381.1992.tb12757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. D., Oon B. B., Thomas C. R., Poston L. Prevention by insulin treatment of endothelial dysfunction but not enhanced noradrenaline-induced contractility in mesenteric resistance arteries from streptozotocin-induced diabetic rats. Br J Pharmacol. 1994 Jan;111(1):35–41. doi: 10.1111/j.1476-5381.1994.tb14020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. D., Wickenden A. D., Mirrlees D. J., Poston L. Endothelial function in the isolated perfused mesentery and aortae of rats with streptozotocin-induced diabetes: effect of treatment with the aldose reductase inhibitor, ponalrestat. Br J Pharmacol. 1994 Jan;111(1):42–48. doi: 10.1111/j.1476-5381.1994.tb14021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Cohen R. A. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991 May;87(5):1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Deykin D., Cohen R. A. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990 Mar;85(3):929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Cohen R. A. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992 Aug;263(2 Pt 2):H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Jakubowski J. A., Cohen R. A. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989 Nov;257(5 Pt 2):H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B. Selective impairment of endothelium-dependent relaxations by prostaglandin endoperoxide. J Hypertens. 1994 Jan;12(1):41–47. [PubMed] [Google Scholar]
- Tooke J. E. Microvascular haemodynamics in diabetes mellitus. Clin Sci (Lond) 1986 Feb;70(2):119–125. doi: 10.1042/cs0700119. [DOI] [PubMed] [Google Scholar]
- Van de Voorde J., Vanheel B., Leusen I. Endothelium-dependent relaxation and hyperpolarization in aorta from control and renal hypertensive rats. Circ Res. 1992 Jan;70(1):1–8. doi: 10.1161/01.res.70.1.1. [DOI] [PubMed] [Google Scholar]
- Vora J. P., Dolben J., Dean J. D., Thomas D., Williams J. D., Owens D. R., Peters J. R. Renal hemodynamics in newly presenting non-insulin dependent diabetes mellitus. Kidney Int. 1992 Apr;41(4):829–835. doi: 10.1038/ki.1992.127. [DOI] [PubMed] [Google Scholar]
- Weidmann P., Beretta-Piccoli C., Keusch G., Glück Z., Mujagic M., Grimm M., Meier A., Ziegler W. H. Sodium-volume factor, cardiovascular reactivity and hypotensive mechanism of diuretic therapy in mild hypertension associated with diabetes mellitus. Am J Med. 1979 Nov;67(5):779–784. doi: 10.1016/0002-9343(79)90734-4. [DOI] [PubMed] [Google Scholar]
- Williams B., Schrier R. W. Characterization of glucose-induced in situ protein kinase C activity in cultured vascular smooth muscle cells. Diabetes. 1992 Nov;41(11):1464–1472. doi: 10.2337/diab.41.11.1464. [DOI] [PubMed] [Google Scholar]


