Abstract
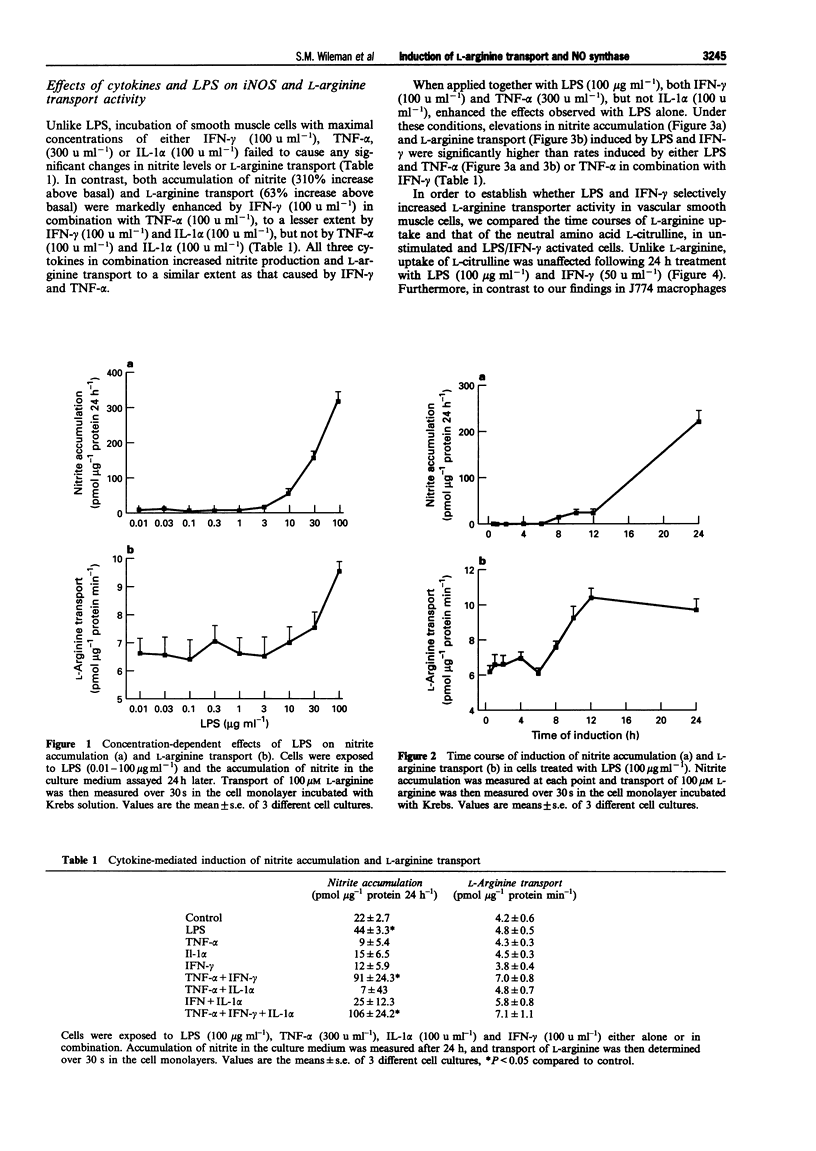
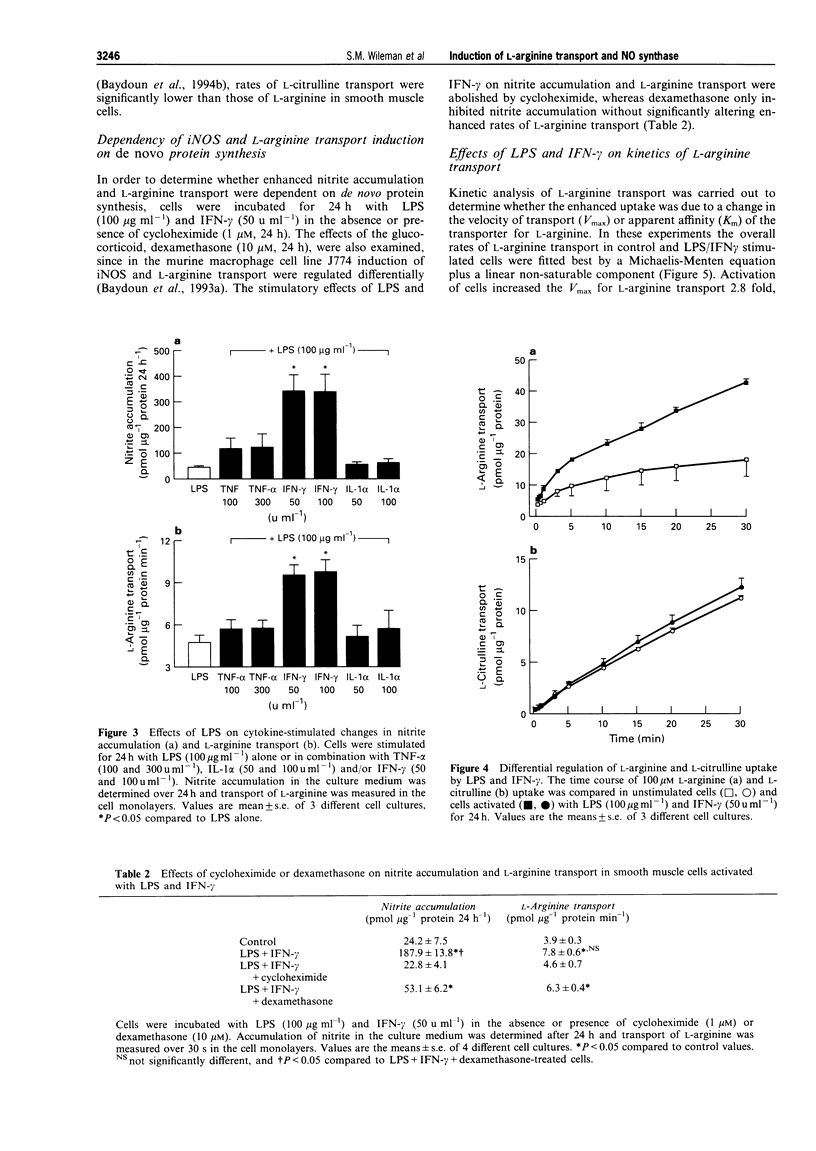
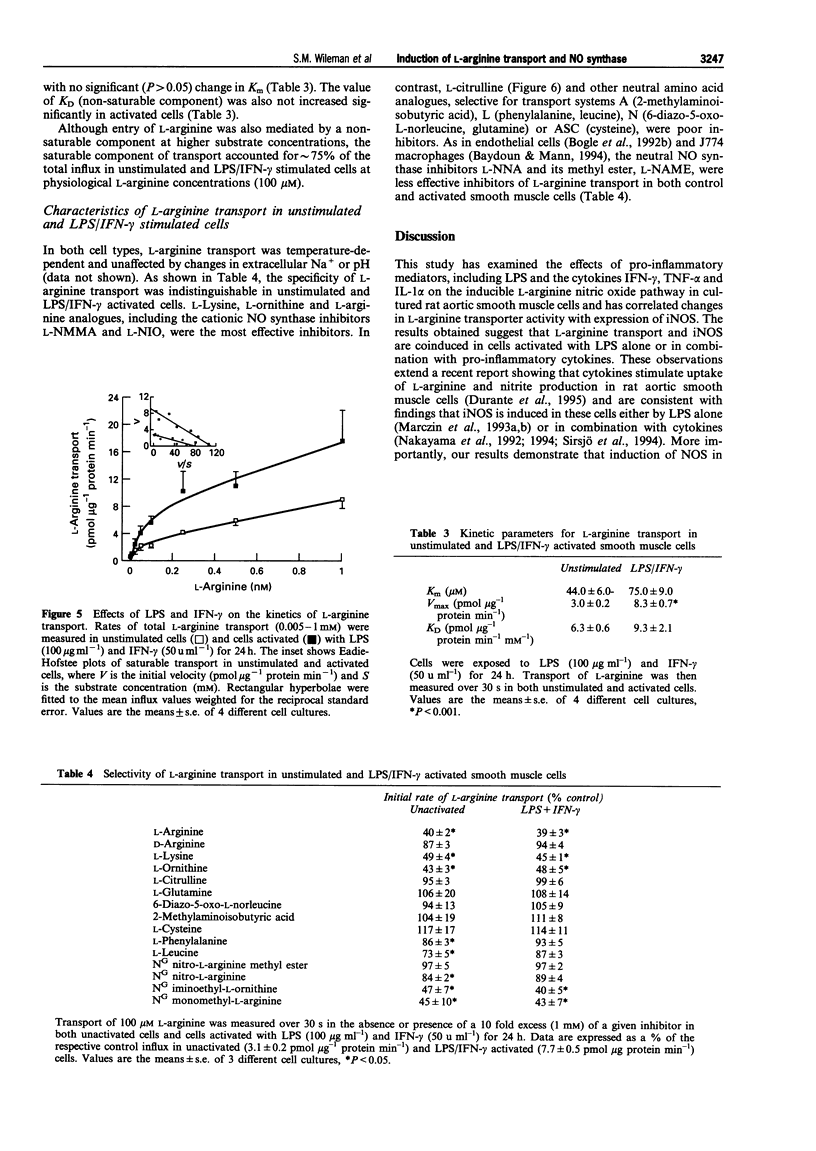
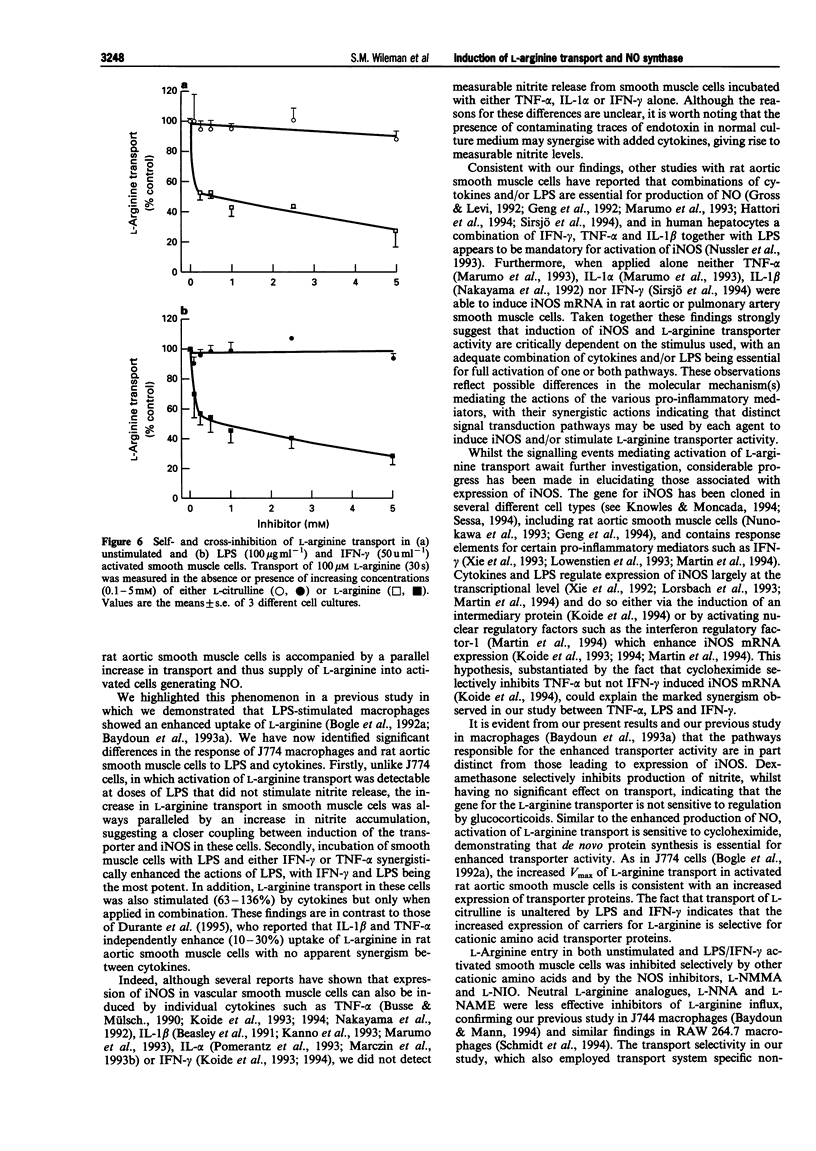
1. The interactions between pro-inflammatory cytokines and bacterial lipopolysaccharide (LPS) on L-arginine transporter and inducible nitric oxide synthase (iNOS) activities were examined in rat cultured aortic smooth muscle cells. 2. LPS induced a concentration (0.01-100 micrograms ml-1) and time (8-24 h)-dependent stimulation of nitrite production which was accompanied by a parallel increase in L-arginine transport. 3. Unlike LPS, activation of smooth muscle cells with either interferon-gamma (IFN-gamma, 100 u ml-1), tumour necrosis factor-alpha (TNF-alpha, 300 u ml-1) or interleukin-1 alpha (IL-1 alpha, 100 u ml-1) failed to stimulate L-arginine transport or increase nitrite accumulation. 4. When applied in combination with LPS (100 micrograms ml-1) both IFN-gamma and TNF-alpha, but not IL-1 alpha, enhanced the effects observed with LPS alone. Furthermore, activation of cells with LPS and IFN-gamma had no effect on uptake of the neutral amino acid L-citrulline but selectively increased the Vmax for L-arginine transport 2.8 fold and nitrite levels from 24 +/- 7 to 188 +/- 14 pmol micrograms-1 protein 24 h-1. 5. The substrate specificity, Na- and pH-independence of saturable L-arginine transport in both unactivated (K(m) = 44 microM, Vmax = 3 pmol micrograms-1 protein min-1) and activated (K(m) = 75 microM, Vmax = 8.3 pmol micrograms-1 protein min-1) smooth muscle cells were characteristic of the cationic amino acid transport system y+. 6. Cycloheximide (1 microM) abolished induction of L-arginine transport and nitrite accumulation in response to LPS and IFN-gamma. In contrast, the glucocorticoid dexamethasone (10 microM, 24 h) selectively inhibited nitrite production. 7. Our results demonstrate that pro-inflammatory mediators selectively enhance transport of L-arginine under conditions of sustained NO synthesis by vascular smooth muscle cells. In addition, the differential inhibition of iNOS and L-arginine transporter activity by dexamethasone suggests that distinct signalling pathways mediate induction of the cationic transport protein and iNOS. The close coupling between substrate supply and NO production may have important implications in the pathogenesis of several disease states including endotoxin shock.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baydoun A. R., Bogle R. G., Pearson J. D., Mann G. E. Discrimination between citrulline and arginine transport in activated murine macrophages: inefficient synthesis of NO from recycling of citrulline to arginine. Br J Pharmacol. 1994 Jun;112(2):487–492. doi: 10.1111/j.1476-5381.1994.tb13099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun A. R., Bogle R. G., Pearson J. D., Mann G. E. Selective inhibition by dexamethasone of induction of NO synthase, but not of induction of L-arginine transport, in activated murine macrophage J774 cells. Br J Pharmacol. 1993 Dec;110(4):1401–1406. doi: 10.1111/j.1476-5381.1993.tb13976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun A. R., Mann G. E. Selective targeting of nitric oxide synthase inhibitors to system y+ in activated macrophages. Biochem Biophys Res Commun. 1994 Apr 29;200(2):726–731. doi: 10.1006/bbrc.1994.1511. [DOI] [PubMed] [Google Scholar]
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogle R. G., Baydoun A. R., Pearson J. D., Moncada S., Mann G. E. L-arginine transport is increased in macrophages generating nitric oxide. Biochem J. 1992 May 15;284(Pt 1):15–18. doi: 10.1042/bj2840015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogle R. G., Moncada S., Pearson J. D., Mann G. E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br J Pharmacol. 1992 Apr;105(4):768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Campbell J. H., Campbell G. R. Culture techniques and their applications to studies of vascular smooth muscle. Clin Sci (Lond) 1993 Nov;85(5):501–513. doi: 10.1042/cs0850501. [DOI] [PubMed] [Google Scholar]
- Closs E. I., Albritton L. M., Kim J. W., Cunningham J. M. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J Biol Chem. 1993 Apr 5;268(10):7538–7544. [PubMed] [Google Scholar]
- Closs E. I., Lyons C. R., Kelly C., Cunningham J. M. Characterization of the third member of the MCAT family of cationic amino acid transporters. Identification of a domain that determines the transport properties of the MCAT proteins. J Biol Chem. 1993 Oct 5;268(28):20796–20800. [PubMed] [Google Scholar]
- Durante W., Liao L., Schafer A. I. Differential regulation of L-arginine transport and inducible NOS in cultured vascular smooth muscle cells. Am J Physiol. 1995 Mar;268(3 Pt 2):H1158–H1164. doi: 10.1152/ajpheart.1995.268.3.H1158. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Julou-Schaeffer G., Parratt J. R., Stoclet J. C. Incubation with endotoxin activates the L-arginine pathway in vascular tissue. Biochem Biophys Res Commun. 1990 Sep 14;171(2):562–568. doi: 10.1016/0006-291x(90)91183-s. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Schott C., Stoclet J. C. Inducible but not constitutive production of nitric oxide by vascular smooth muscle cells. Eur J Pharmacol. 1991 Aug 6;200(2-3):375–376. doi: 10.1016/0014-2999(91)90602-m. [DOI] [PubMed] [Google Scholar]
- Geng Y. J., Almqvist M., Hansson G. K. cDNA cloning and expression of inducible nitric oxide synthase from rat vascular smooth muscle cells. Biochim Biophys Acta. 1994 Aug 2;1218(3):421–424. doi: 10.1016/0167-4781(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Geng Y., Hansson G. K., Holme E. Interferon-gamma and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ Res. 1992 Nov;71(5):1268–1276. doi: 10.1161/01.res.71.5.1268. [DOI] [PubMed] [Google Scholar]
- Gray G. A., Schott C., Julou-Schaeffer G., Fleming I., Parratt J. R., Stoclet J. C. The effect of inhibitors of the L-arginine/nitric oxide pathway on endotoxin-induced loss of vascular responsiveness in anaesthetized rats. Br J Pharmacol. 1991 May;103(1):1218–1224. doi: 10.1111/j.1476-5381.1991.tb12327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem. 1992 Dec 25;267(36):25722–25729. [PubMed] [Google Scholar]
- Harper J. F. Peritz' F test: basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput Biol Med. 1984;14(4):437–445. doi: 10.1016/0010-4825(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Campbell E. B., Gross S. S. Argininosuccinate synthetase mRNA and activity are induced by immunostimulants in vascular smooth muscle. Role in the regeneration or arginine for nitric oxide synthesis. J Biol Chem. 1994 Apr 1;269(13):9405–9408. [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kanno K., Hirata Y., Imai T., Marumo F. Induction of nitric oxide synthase gene by interleukin in vascular smooth muscle cells. Hypertension. 1993 Jul;22(1):34–39. doi: 10.1161/01.hyp.22.1.34. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Koide M., Kawahara Y., Tsuda T., Yokoyama M. Cytokine-induced expression of an inducible type of nitric oxide synthase gene in cultured vascular smooth muscle cells. FEBS Lett. 1993 Mar 8;318(3):213–217. doi: 10.1016/0014-5793(93)80514-u. [DOI] [PubMed] [Google Scholar]
- Lorsbach R. B., Murphy W. J., Lowenstein C. J., Snyder S. H., Russell S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993 Jan 25;268(3):1908–1913. [PubMed] [Google Scholar]
- Low B. C., Ross I. K., Grigor M. R. Characterization of system L and system y+ amino acid transport activity in cultured vascular smooth muscle cells. J Cell Physiol. 1993 Sep;156(3):626–634. doi: 10.1002/jcp.1041560323. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Alley E. W., Raval P., Snowman A. M., Snyder S. H., Russell S. W., Murphy W. J. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczin N., Papapetropoulos A., Catravas J. D. Tyrosine kinase inhibitors suppress endotoxin- and IL-1 beta-induced NO synthesis in aortic smooth muscle cells. Am J Physiol. 1993 Sep;265(3 Pt 2):H1014–H1018. doi: 10.1152/ajpheart.1993.265.3.H1014. [DOI] [PubMed] [Google Scholar]
- Marczin N., Papapetropoulos A., Jilling T., Catravas J. D. Prevention of nitric oxide synthase induction in vascular smooth muscle cells by microtubule depolymerizing agents. Br J Pharmacol. 1993 Jul;109(3):603–605. doi: 10.1111/j.1476-5381.1993.tb13613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Nathan C., Xie Q. W. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med. 1994 Sep 1;180(3):977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo T., Nakaki T., Nagata K., Miyata M., Adachi H., Esumi H., Suzuki H., Saruta T., Kato R. Dexamethasone inhibits nitric oxide synthase mRNA induction by interleukin-1 alpha and tumor necrosis factor-alpha in vascular smooth muscle cells. Jpn J Pharmacol. 1993 Nov;63(3):361–367. doi: 10.1254/jjp.63.361. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nakayama D. K., Geller D. A., Di Silvio M., Bloomgarden G., Davies P., Pitt B. R., Hatakeyama K., Kagamiyama H., Simmons R. L., Billiar T. R. Tetrahydrobiopterin synthesis and inducible nitric oxide production in pulmonary artery smooth muscle. Am J Physiol. 1994 Apr;266(4 Pt 1):L455–L460. doi: 10.1152/ajplung.1994.266.4.L455. [DOI] [PubMed] [Google Scholar]
- Nakayama D. K., Geller D. A., Lowenstein C. J., Chern H. D., Davies P., Pitt B. R., Simmons R. L., Billiar T. R. Cytokines and lipopolysaccharide induce nitric oxide synthase in cultured rat pulmonary artery smooth muscle. Am J Respir Cell Mol Biol. 1992 Nov;7(5):471–476. doi: 10.1165/ajrcmb/7.5.471. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group report on hypertension in diabetes. Hypertension. 1994 Feb;23(2):145–160. [PubMed] [Google Scholar]
- Nunokawa Y., Ishida N., Tanaka S. Cloning of inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Feb 26;191(1):89–94. doi: 10.1006/bbrc.1993.1188. [DOI] [PubMed] [Google Scholar]
- Nüssler A. K., Geller D. A., Sweetland M. A., Di Silvio M., Billiar T. R., Madariaga J. B., Simmons R. L., Lancaster J. R., Jr Induction of nitric oxide synthesis and its reactions in cultured human and rat hepatocytes stimulated with cytokines plus LPS. Biochem Biophys Res Commun. 1993 Jul 30;194(2):826–835. doi: 10.1006/bbrc.1993.1896. [DOI] [PubMed] [Google Scholar]
- Pomerantz K. B., Hajjar D. P., Levi R., Gross S. S. Cholesterol enrichment of arterial smooth muscle cells upregulates cytokine-induced nitric oxide synthesis. Biochem Biophys Res Commun. 1993 Feb 26;191(1):103–109. doi: 10.1006/bbrc.1993.1190. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Klatt P., Mayer B. Uptake of nitric oxide synthase inhibitors by macrophage RAW 264.7 cells. Biochem J. 1994 Jul 15;301(Pt 2):313–316. doi: 10.1042/bj3010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott C. A., Gray G. A., Stoclet J. C. Dependence of endotoxin-induced vascular hyporeactivity on extracellular L-arginine. Br J Pharmacol. 1993 Jan;108(1):38–43. doi: 10.1111/j.1476-5381.1993.tb13436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa W. C. The nitric oxide synthase family of proteins. J Vasc Res. 1994 May-Jun;31(3):131–143. doi: 10.1159/000159039. [DOI] [PubMed] [Google Scholar]
- Sirsjö A., Söderkvist P., Sundqvist T., Carlsson M., Ost M., Gidlöf A. Different induction mechanisms of mRNA for inducible nitric oxide synthase in rat smooth muscle cells in culture and in aortic strips. FEBS Lett. 1994 Jan 31;338(2):191–196. doi: 10.1016/0014-5793(94)80363-3. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]


