Abstract
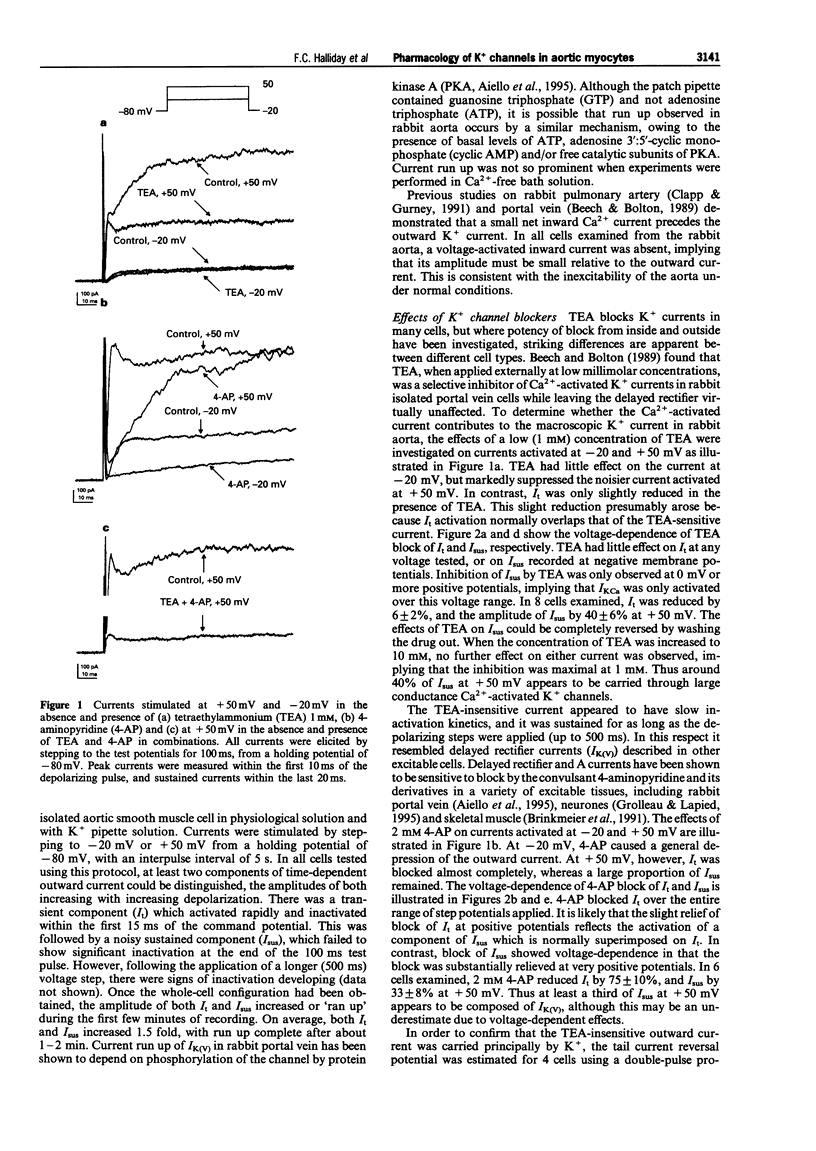
1. Using the whole-cell patch-clamp technique, the effects of several K+ channel blocking drugs on K+ current recorded from rabbit isolated aortic smooth muscle cells were investigated. 2. Upon depolarization from -80 mV, outward K+ current composed of several distinct components were observed: a transient, 4-aminopyridine (4-AP)-sensitive component (I1) and a sustained component (Isus), comprising a 4-AP-sensitive delayed rectifier current (IK(V)), and a noisy current which was sensitive to tetraethylammonium (TEA), and probably due to Ca(2+)-activated K+ current (IK(Ca)). 3. Several drugs in clinical or experimental use have as part of their action an inhibitory effect on specific K+ channels. Because of their differential K+ channel blocking effects, these drugs were used in an attempt to characterize further the K+ channels in rabbit aortic smooth muscle cells. Imipramine, phencyclidine, sotalol and amitriptyline failed to block selectively any of the components of K+ current, and were thus of little value in isolating individual channel contributions. Clofilium showed selective block of IK(V) in the presence of TEA, but only at low stimulation frequencies (0.07 Hz). At higher frequencies (1 Hz) of depolarization, both I1 and IK(V) were suppressed to a similar extent. Thus, the blocking action of clofilium was use-dependent. 4. The voltage-dependent inactivation of I1 and the delayed rectifier were very similar although a brief (100 ms) pre-pulse to -30 mV could preferentially inactivate I1. Together with the non-selective blocking effects of the K+ channel blockers, similarities in the activation and inactivation of these two components suggest that they may not exist as separate ionic channels, but as distinct kinetic states within the same K+ channel population. 5. The effects of all of these drugs on tension were examined in strips of rabbit aorta. The non-specific K+ channel blockers caused only minor increases in basal tension. TEA and 4-AP by themselves caused significant increases in tension and were even more effective when applied together. There appeared to be no correlation between the effects of the drugs tested on tension and their actions on currents recorded from isolated myocytes. Thus tension studies are an inappropriate means of investigating the mechanism of action of these drugs, and studies on ionic currents in isolated myocytes cannot easily predict drug actions on intact tissues.
Full text
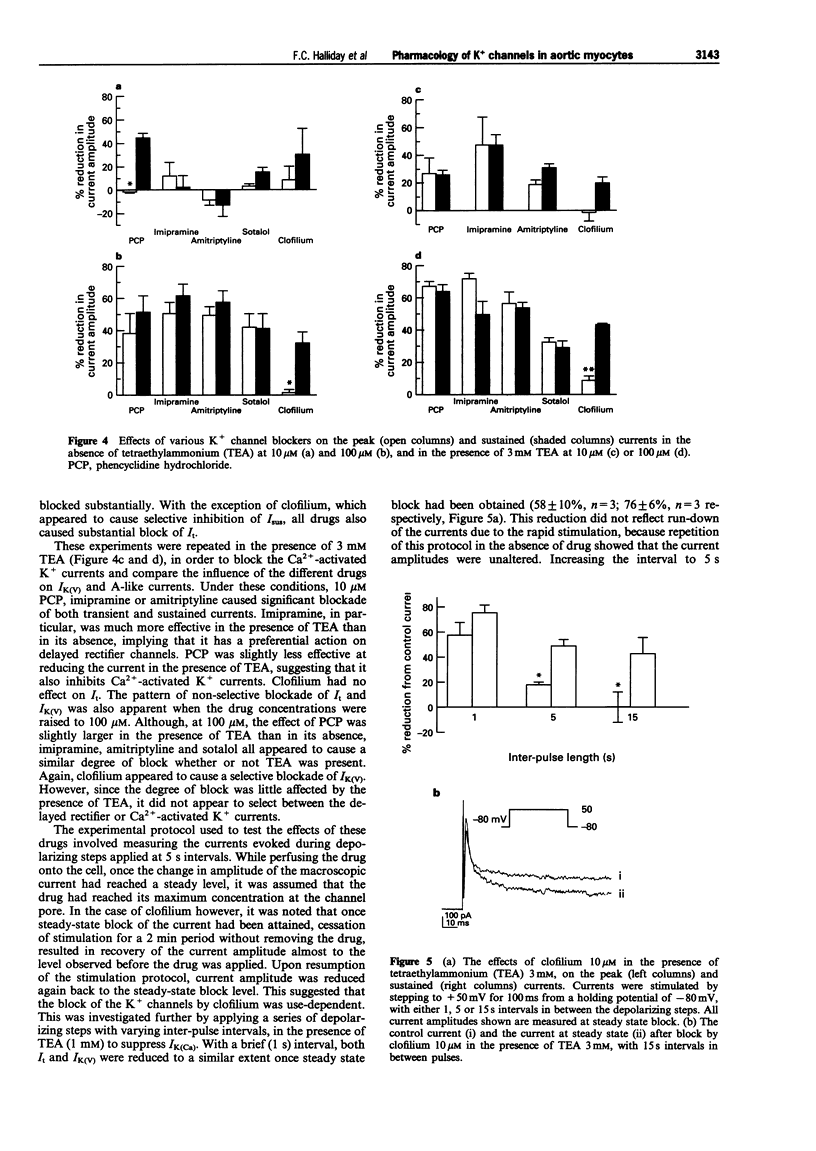
PDF


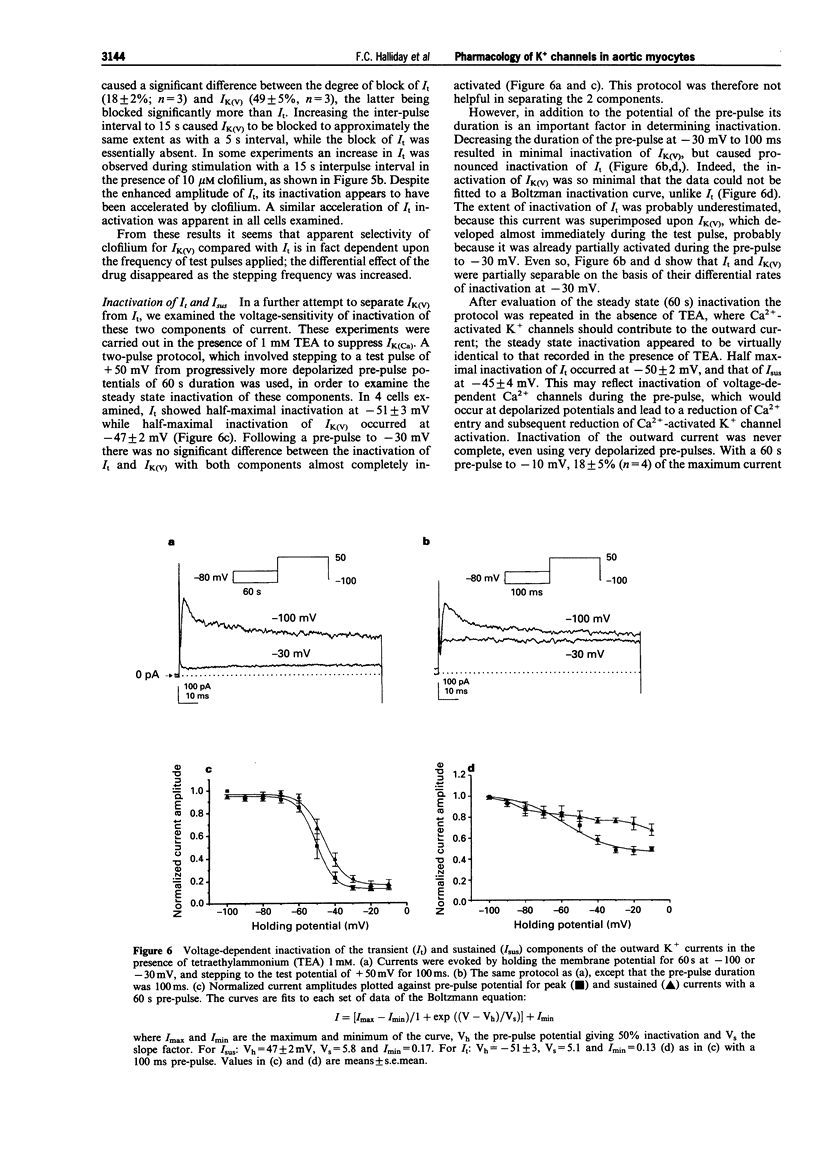



Selected References
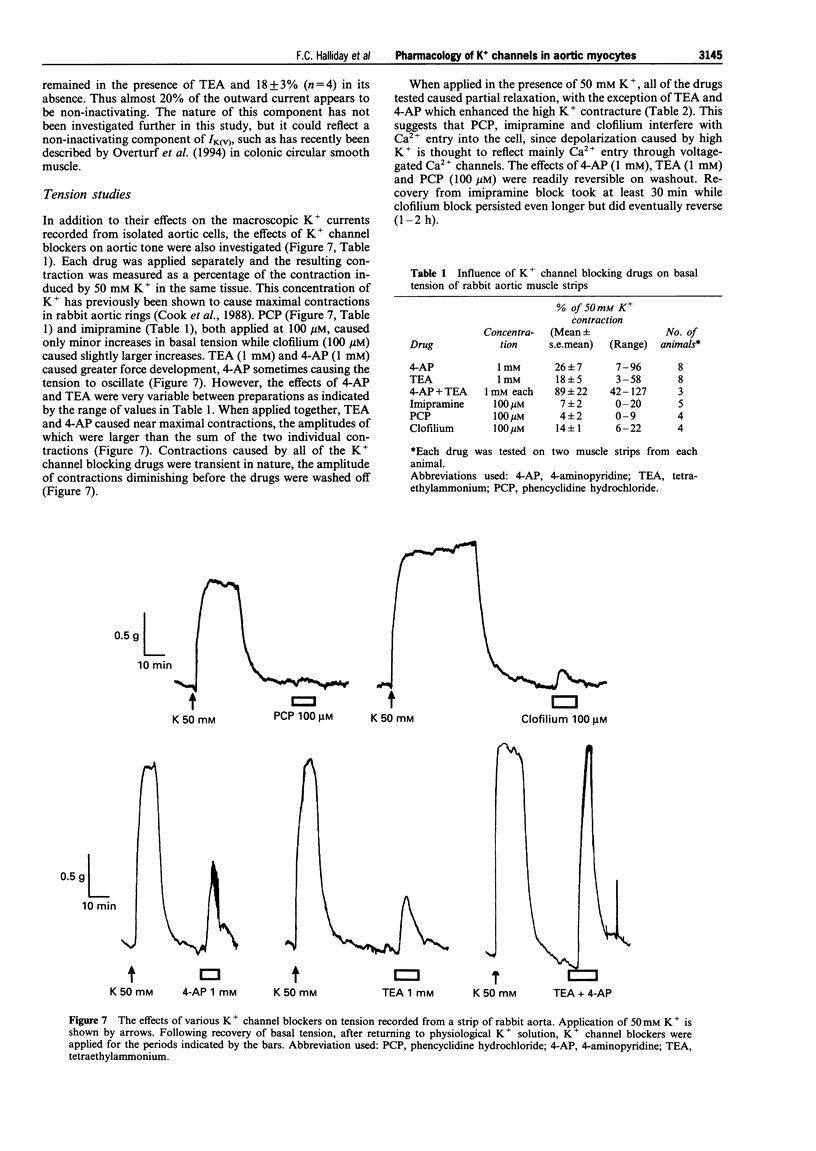
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello E. A., Walsh M. P., Cole W. C. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995 Feb;268(2 Pt 2):H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- Arena J. P., Kass R. S. Block of heart potassium channels by clofilium and its tertiary analogs: relationship between drug structure and type of channel blocked. Mol Pharmacol. 1988 Jul;34(1):60–66. [PubMed] [Google Scholar]
- Beckh S., Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J. 1990 Mar;9(3):777–782. doi: 10.1002/j.1460-2075.1990.tb08173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T., Benham C. D. Patch and whole-cell voltage clamp of single mammalian visceral and vascular smooth muscle cells. Experientia. 1985 Jul 15;41(7):887–894. doi: 10.1007/BF01970006. [DOI] [PubMed] [Google Scholar]
- Brinkmeier H., Zachar E., Rüdel R. Voltage-dependent K+ channels in the sarcolemma of mouse skeletal muscle. Pflugers Arch. 1991 Nov;419(5):486–491. doi: 10.1007/BF00370793. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Electrophysiologic and voltage clamp analysis of the effects of sotalol on isolated cardiac muscle and Purkinje fibers. J Pharmacol Exp Ther. 1985 Mar;232(3):817–825. [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauvin C., Lukeman S., Cameron J., Hwang O., Meisheri K., Yamamoto H., van Breemen C. Theoretical bases for vascular selectivity of Ca2+ antagonists. J Cardiovasc Pharmacol. 1984;6 (Suppl 4):S630–S638. doi: 10.1097/00005344-198406004-00009. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991 Sep;76(5):677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Weir S. W., Danzeisen M. C. Anti-vasoconstrictor effects of the K+ channel opener cromakalim on the rabbit aorta--comparison with the calcium antagonist isradipine. Br J Pharmacol. 1988 Nov;95(3):741–752. doi: 10.1111/j.1476-5381.1988.tb11700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpón E., Tamargo J., Sánchez-Chapula J. Effects of imipramine on the transient outward current in rabbit atrial single cells. Br J Pharmacol. 1992 Jun;106(2):464–469. doi: 10.1111/j.1476-5381.1992.tb14357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh W. J., Gibson K. J., McAnulty J. H., Maylie J. G. Beta-adrenergic blocking property of dl-sotalol maintains class III efficacy in guinea pig ventricular muscle after isoproterenol. Circulation. 1995 Jan 15;91(2):262–264. doi: 10.1161/01.cir.91.2.262. [DOI] [PubMed] [Google Scholar]
- Grolleau F., Lapied B. Separation and identification of multiple potassium currents regulating the pacemaker activity of insect neurosecretory cells (DUM neurons). J Neurophysiol. 1995 Jan;73(1):160–171. doi: 10.1152/jn.1995.73.1.160. [DOI] [PubMed] [Google Scholar]
- Hadley R. W., Hume J. R. Actions of phencyclidine on the action potential and membrane currents of single guinea-pig myocytes. J Pharmacol Exp Ther. 1986 Apr;237(1):131–136. [PubMed] [Google Scholar]
- Haeusler G., De Peyer J. E. Rabbit aorta: electrical properties and agonist-induced depolarization. Eur J Pharmacol. 1989 Jul 18;166(2):175–182. doi: 10.1016/0014-2999(89)90057-5. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Mikala G., Yatani A. A novel enhancing effect of clofilium on transient outward-type cloned cardiac K+ channel currents. Br J Pharmacol. 1995 Mar;114(6):1222–1226. doi: 10.1111/j.1476-5381.1995.tb13336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malayev A. A., Nelson D. J., Philipson L. H. Mechanism of clofilium block of the human Kv1.5 delayed rectifier potassium channel. Mol Pharmacol. 1995 Jan;47(1):198–205. [PubMed] [Google Scholar]
- Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974 Oct;242(1):143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon C. B., Avdonin P. V., Larsen M. A., Bobik A. Rat aortic smooth muscle cells expressing charybdotoxin-sensitive potassium channels exhibit enhanced proliferative responses. Clin Exp Pharmacol Physiol. 1994 Feb;21(2):117–120. doi: 10.1111/j.1440-1681.1994.tb02477.x. [DOI] [PubMed] [Google Scholar]
- Overturf K. E., Russell S. N., Carl A., Vogalis F., Hart P. J., Hume J. R., Sanders K. M., Horowitz B. Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am J Physiol. 1994 Nov;267(5 Pt 1):C1231–C1238. doi: 10.1152/ajpcell.1994.267.5.C1231. [DOI] [PubMed] [Google Scholar]
- Po S., Roberds S., Snyders D. J., Tamkun M. M., Bennett P. B. Heteromultimeric assembly of human potassium channels. Molecular basis of a transient outward current? Circ Res. 1993 Jun;72(6):1326–1336. doi: 10.1161/01.res.72.6.1326. [DOI] [PubMed] [Google Scholar]
- Reeve H. L., Peers C. Blockade of delayed rectifier K+ currents in neuroblastoma x glioma hybrid (NG 108-15) cells by clofilium, a class III antidysrhythmic agent. Br J Pharmacol. 1992 Feb;105(2):458–462. doi: 10.1111/j.1476-5381.1992.tb14275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. N., Overturf K. E., Horowitz B. Heterotetramer formation and charybdotoxin sensitivity of two K+ channels cloned from smooth muscle. Am J Physiol. 1994 Dec;267(6 Pt 1):C1729–C1733. doi: 10.1152/ajpcell.1994.267.6.C1729. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C. Modulation of potassium channels by antiarrhythmic and antihypertensive drugs. Hypertension. 1992 Mar;19(3):228–236. doi: 10.1161/01.hyp.19.3.228. [DOI] [PubMed] [Google Scholar]
- Smirnov S. V., Aaronson P. I. Ca(2+)-activated and voltage-gated K+ currents in smooth muscle cells isolated from human mesenteric arteries. J Physiol. 1992 Nov;457:431–454. doi: 10.1113/jphysiol.1992.sp019386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Cook D. Cerebral vascular smooth muscle potassium channels and their possible role in the management of vasospasm. Pharmacol Toxicol. 1994 Dec;75(6):327–336. doi: 10.1111/j.1600-0773.1994.tb00370.x. [DOI] [PubMed] [Google Scholar]


