Abstract
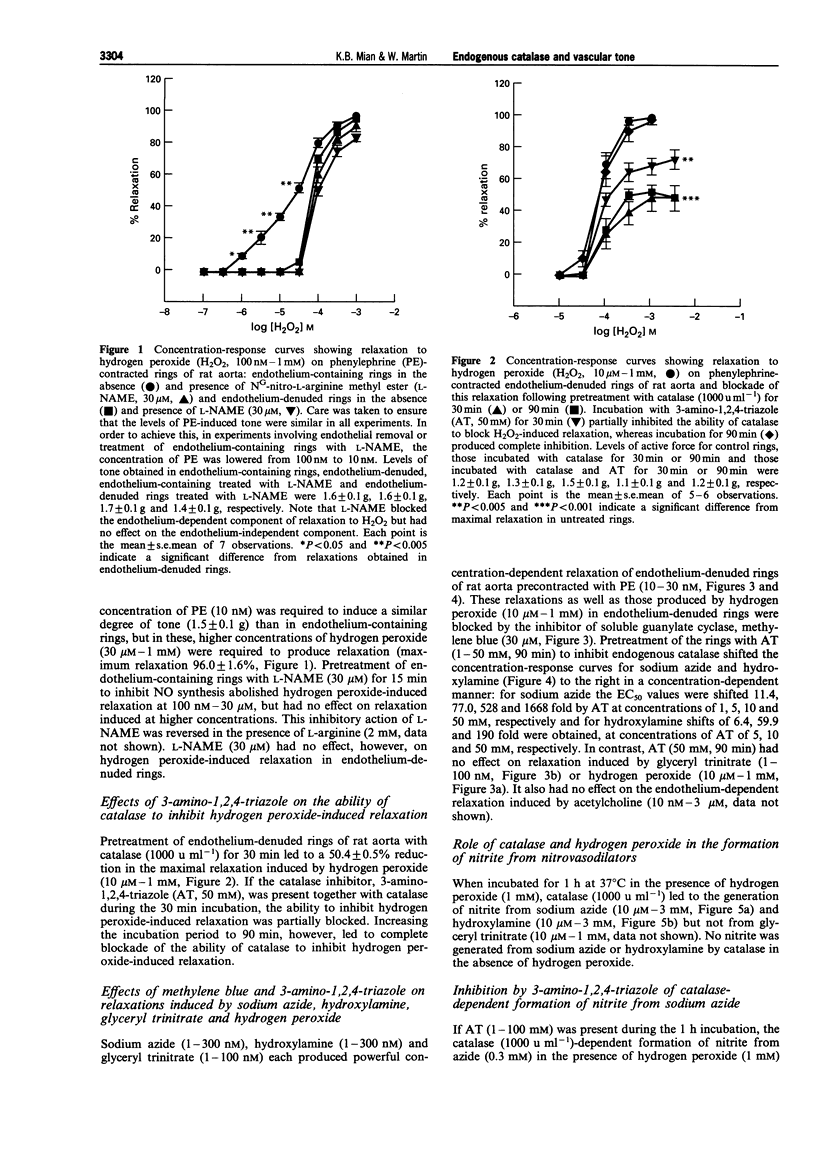
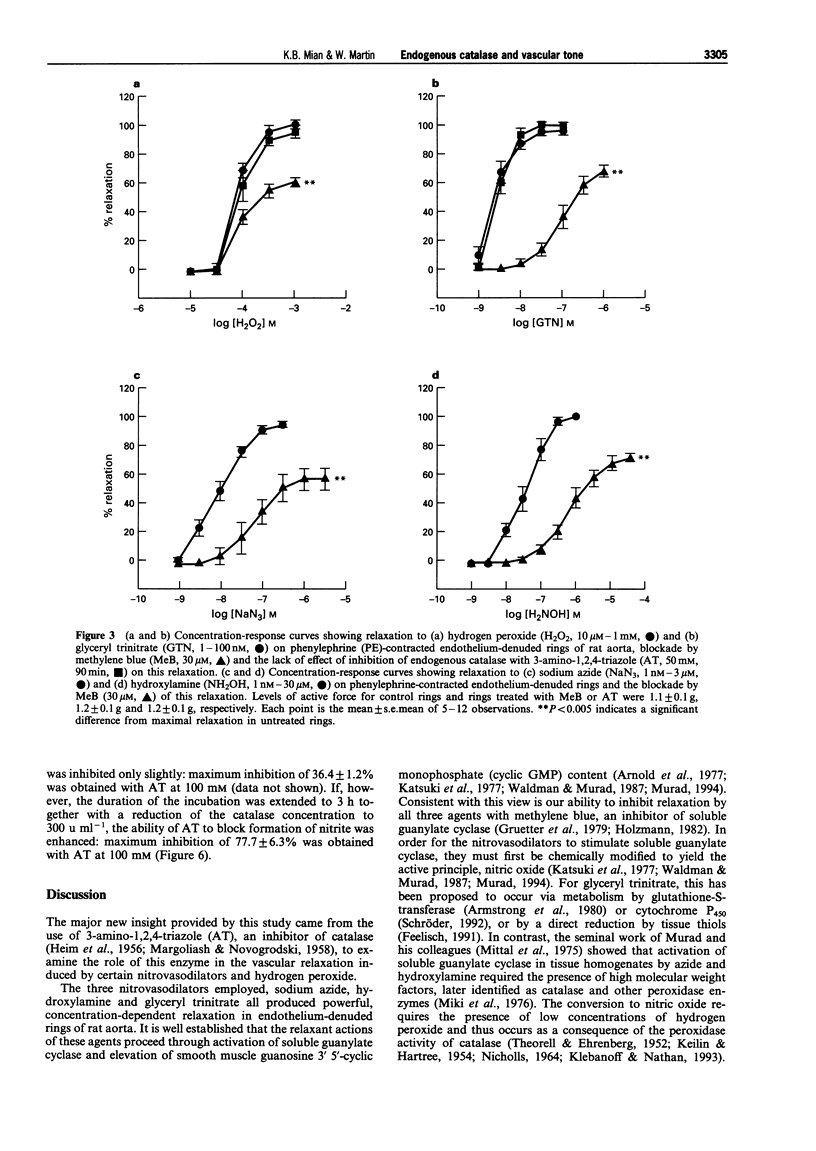
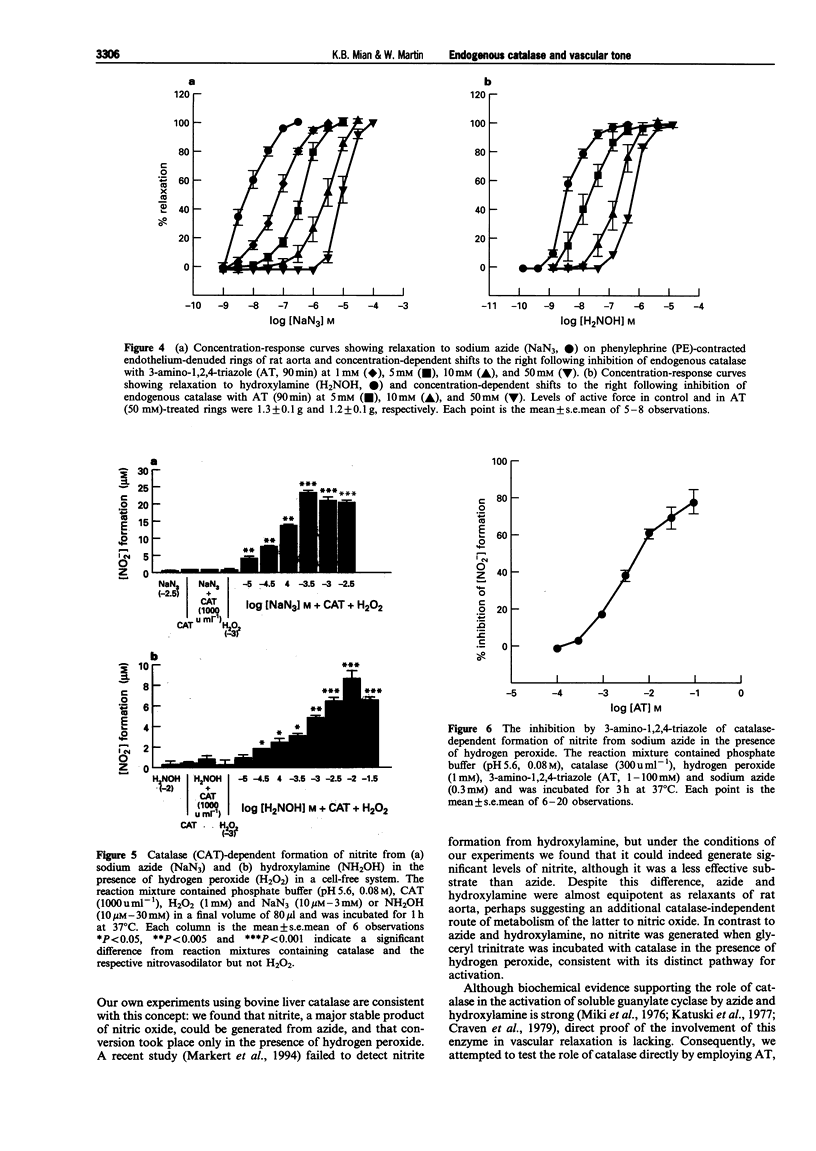
1. In this study we investigated the role of catalase in relaxation induced by hydroxylamine, sodium azide, glyceryl trinitrate and hydrogen peroxide in isolated rings of rat aorta. 2. Hydrogen peroxide (1 microM-1 mM)-induced concentration-dependent relaxation of phenylephrine (PE)-induced tone in endothelium-containing rings. In endothelium-denuded rings, however, higher concentrations (30 microM-1 mM) of hydrogen peroxide were required to produce relaxation. The endothelium-dependent component of hydrogen peroxide-induced relaxation was abolished following pretreatment with N(O)-nitro-L-arginine methyl ester (L-NAME, 30 microM). L-NAME (30 microM) had no effect, however, on hydrogen peroxide-induced relaxation in endothelium-denuded rings. 3. Pretreatment of endothelium-denuded rings with catalase (1000 u ml-1) blocked relaxation induced by hydrogen peroxide (10 microM-1 mM). The ability of catalase to inhibit hydrogen peroxide-induced relaxation was partially blocked following incubation with 3-amino-1,2, 4-triazole (AT, 50 mM) for 30 min and completely blocked at 90 min. 4. Pretreatment of endothelium-denuded rings with methylene blue (MeB, 30 microM) inhibited relaxation induced by hydrogen peroxide (10 microM-1 mM), sodium azide (1-300 nM), hydroxylamine (1-300 nM) and glyceryl trinitrate (1-100 nM) suggesting that each acted by stimulation of soluble guanylate cyclase. 5. Pretreatment of endothelium-denuded rings with AT (1-50 mM, 90 min) to inhibit endogenous catalase blocked relaxation induced by sodium azide (1-300 nM) and hydroxylamine (1-300 nM) but had no effect on relaxation induced by hydrogen peroxide (10 microM-1 mM) or glyceryl trinitrate (1-100 nM). 6. In a cell-free system, incubation of sodium azide (10 microM-3 mM) and hydroxylamine (10 microM-30 mM) but not glyceryl trinitrate (10 microM-1 mM) with catalase (1000 u ml-1) in the presence of hydrogen peroxide (1 mM) led to production of nitrite, a major breakdown product of nitric oxide. AT (1-100 mM) inhibited, in a concentration-dependent manner, the formation of nitrite from azide in the presence of hydrogen peroxide. 7. These data suggest that metabolism by catalase plays an important role in the relaxation induced by hydroxylamine and sodium azide in isolated rings of rat aorta. Relaxation appears to be due to formation of nitric oxide and activation of soluble guanylate cyclase. In contrast, metabolism by catalase does not appear to be involved in the relaxant actions of hydrogen peroxide or glyceryl trinitrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEMAN D., HEIM W. G., PYFROM H. T. Effects of 3-amino-1, 2, 4-triazole (AT) on catalase and other compounds. Am J Physiol. 1956 Jul;186(1):19–23. doi: 10.1152/ajplegacy.1956.186.1.19. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Slaughter S. E., Marks G. S., Armstrong P. W. Rapid disappearance of nitroglycerin following incubation with human blood. Can J Physiol Pharmacol. 1980 May;58(5):459–462. doi: 10.1139/y80-077. [DOI] [PubMed] [Google Scholar]
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. M., Wolin M. S. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol. 1987 Apr;252(4 Pt 2):H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- CHANCE B., GREENSTEIN D. S., ROUGHTON F. J. W. The mechanism of catalase action. I. Steady-state analysis. Arch Biochem Biophys. 1952 Jun;37(2):301–321. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]
- Cheeseman K. H., Slater T. F. An introduction to free radical biochemistry. Br Med Bull. 1993 Jul;49(3):481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R., Pratt D. W. Electron spin resonance study of the role of NO . catalase in the activation of guanylate cyclase by NaN3 and NH2OH. Modulation of enzyme responses by heme proteins and their nitrosyl derivatives. J Biol Chem. 1979 Sep 10;254(17):8213–8222. [PubMed] [Google Scholar]
- DeMaster E. G., Raij L., Archer S. L., Weir E. K. Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of L-arginine to nitric oxide. Biochem Biophys Res Commun. 1989 Aug 30;163(1):527–533. doi: 10.1016/0006-291x(89)92169-4. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Halliwell B. Production of superoxide, hydrogen peroxide and hydroxyl radicals by phagocytic cells: a cause of chronic inflammatory disease? Cell Biol Int Rep. 1982 Jun;6(6):529–542. doi: 10.1016/0309-1651(82)90175-8. [DOI] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Reactions of methaemoglobin and catalase with peroxides and hydrogen donors. Nature. 1954 Apr 17;173(4407):720–723. doi: 10.1038/173720a0. [DOI] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Nathan C. F. Nitrite production by stimulated human polymorphonuclear leukocytes supplemented with azide and catalase. Biochem Biophys Res Commun. 1993 Nov 30;197(1):192–196. doi: 10.1006/bbrc.1993.2459. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958 Mar;68(3):468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert M., Carnal B., Mauël J. Nitric oxide production by activated human neutrophils exposed to sodium azide and hydroxylamine: the role of oxygen radicals. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1245–1249. doi: 10.1006/bbrc.1994.1364. [DOI] [PubMed] [Google Scholar]
- Miki N., Nagano M., Kuriyama K. Catalase activates cerebral granylate cyclase in the presence of sodium azide. Biochem Biophys Res Commun. 1976 Oct 4;72(3):952–959. doi: 10.1016/s0006-291x(76)80224-0. [DOI] [PubMed] [Google Scholar]
- Mittal C. K., Kimura H., Murad F. Requirement for a macromolecular factor for sodium azide activation of guanulate cyclase. J Cyclic Nucleotide Res. 1975;1(6):261–269. [PubMed] [Google Scholar]
- Murad F. Regulation of cytosolic guanylyl cyclase by nitric oxide: the NO-cyclic GMP signal transduction system. Adv Pharmacol. 1994;26:19–33. doi: 10.1016/s1054-3589(08)60049-6. [DOI] [PubMed] [Google Scholar]
- Nicholls P. The reaction of azide with catalase and their significance. Biochem J. 1964 Feb;90(2):331–343. doi: 10.1042/bj0900331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Zernikow B., Baeblich S., Böhme E. Basal and stimulated formation and release of L-arginine-derived nitrogen oxides from cultured endothelial cells. J Pharmacol Exp Ther. 1990 Aug;254(2):591–597. [PubMed] [Google Scholar]
- Schröder H. Cytochrome P-450 mediates bioactivation of organic nitrates. J Pharmacol Exp Ther. 1992 Jul;262(1):298–302. [PubMed] [Google Scholar]
- Smith J. K., Grisham M. B., Granger D. N., Korthuis R. J. Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am J Physiol. 1989 Mar;256(3 Pt 2):H789–H793. doi: 10.1152/ajpheart.1989.256.3.H789. [DOI] [PubMed] [Google Scholar]
- THEORELL H., EHRENBERG A. The reaction between catalase, azide and hydrogen peroxide. Arch Biochem Biophys. 1952 Dec;41(2):442–474. [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- White A. A., Crawford K. M., Patt C. S., Lad P. J. Activation of soluble guanylate cyclase from rat lung by incubation or by hydrogen peroxide. J Biol Chem. 1976 Dec 10;251(23):7304–7312. [PubMed] [Google Scholar]
- Wolin M. S., Burke T. M. Hydrogen peroxide elicits activation of bovine pulmonary arterial soluble guanylate cyclase by a mechanism associated with its metabolism by catalase. Biochem Biophys Res Commun. 1987 Feb 27;143(1):20–25. doi: 10.1016/0006-291x(87)90623-1. [DOI] [PubMed] [Google Scholar]
- Zembowicz A., Hatchett R. J., Jakubowski A. M., Gryglewski R. J. Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br J Pharmacol. 1993 Sep;110(1):151–158. doi: 10.1111/j.1476-5381.1993.tb13785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


