Abstract
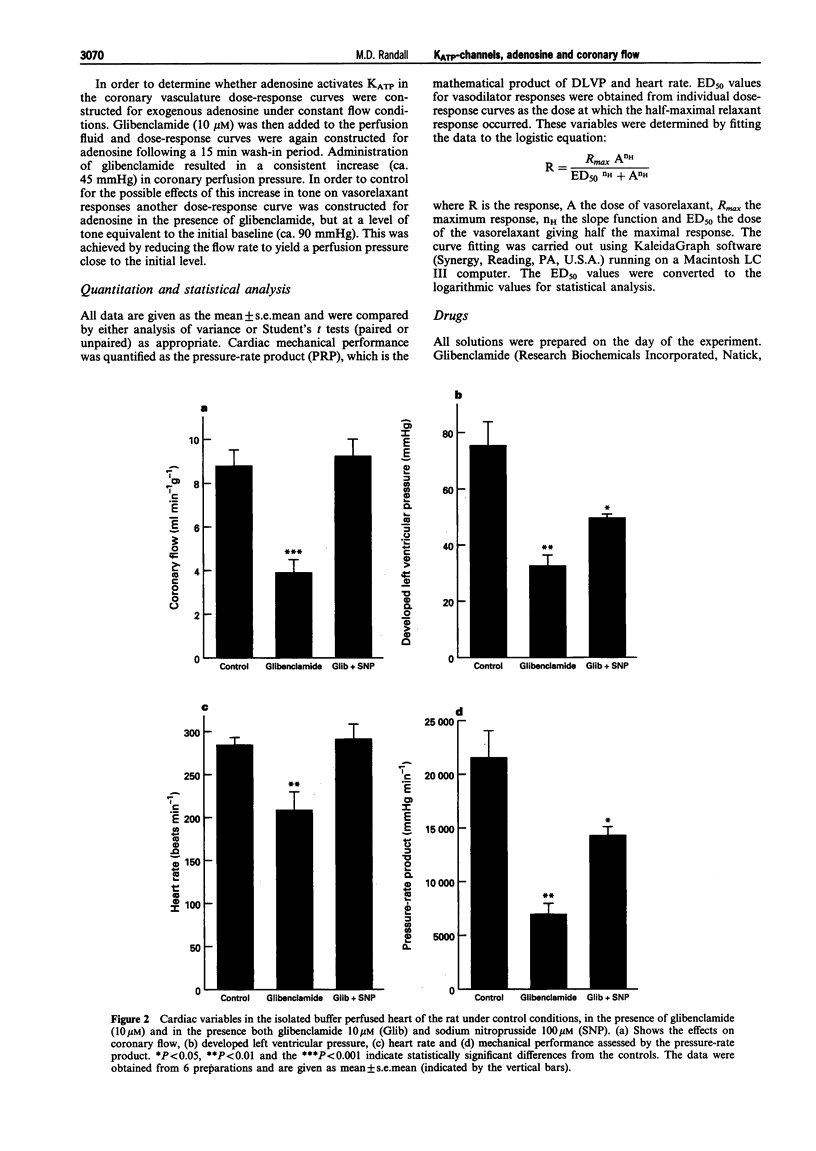
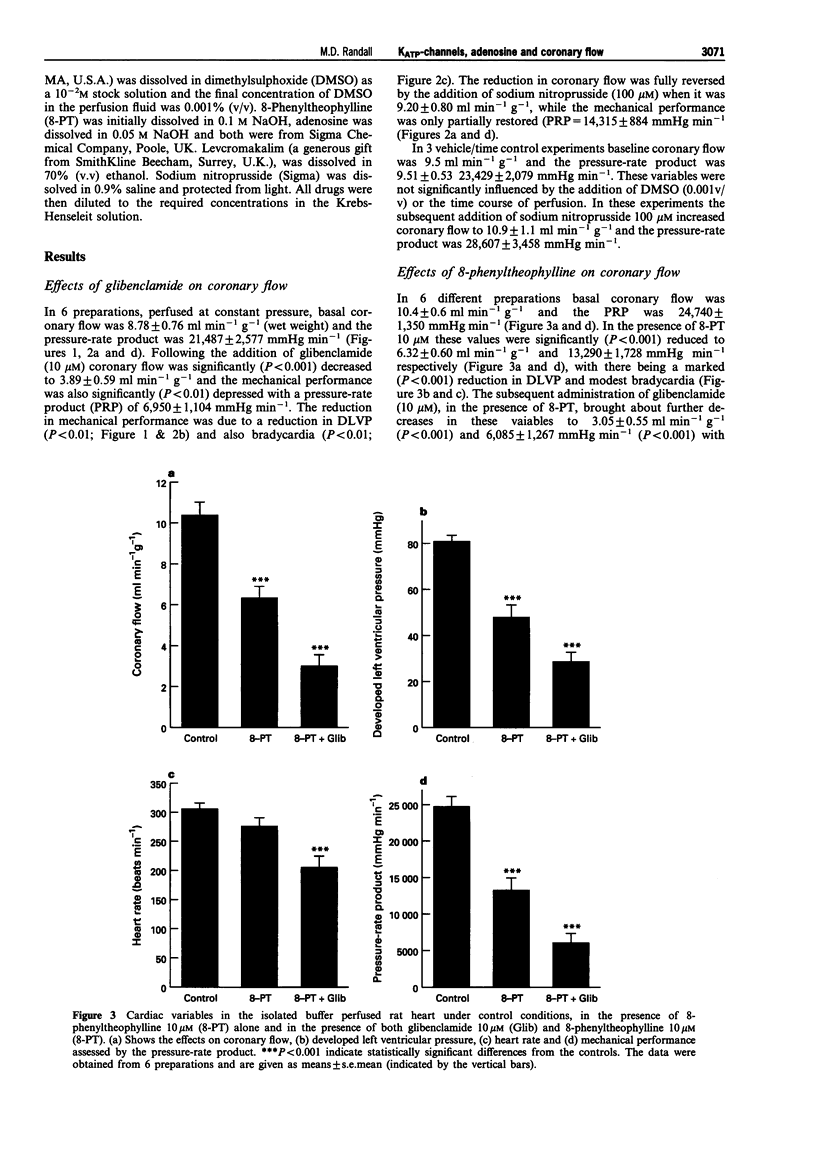
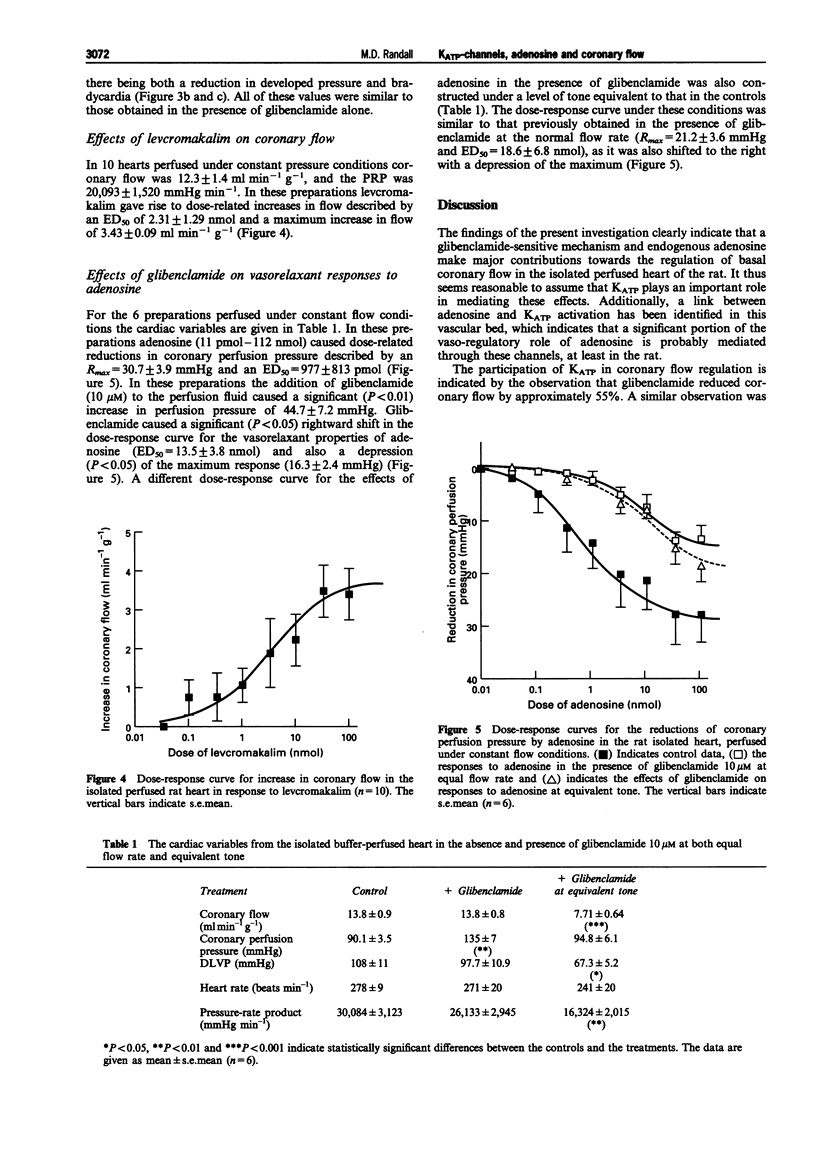
1. The roles of adenosine 5'-triphosphate (ATP)-sensitive potassium channels (KATP) and endogenous adenosine in the regulation of coronary flow have been assessed in the isolated, buffer-perfused heart of the rat. 2. In the presence of glibenclamide 10 microM there was a significant (P < 0.001) reduction in coronary flow from a baseline value of 8.78 +/- 0.76 ml min-1 g-1 to 3.89 +/- 0.59 ml min-1 g-1. This change was accompanied by a significant (P < 0.01) reduction in cardiac mechanical performance as shown by the decrease in the pressure-rate product from 21,487 +/- 2,577 mmHg min-1 to 6,950 +/- 1,104 mmHg min-1. 3. The non-selective adenosine antagonist 8-phenyltheophylline (10 microM) also caused a significant (P < 0.001) reduction in coronary flow from a basal value of 10.4 +/- 0.6 ml min-1 g-1 to 6.32 +/- 0.60 ml min-1 g-1. The subsequent addition of glibenclamide, in the presence of 8-phenyltheophylline, brought about a further significant (P < 0.001) reduction in coronary flow to 3.05 +/- 0.55 ml min-1 g-1 and this value was similar to that in the presence of glibenclamide alone. 4. In hearts perfused under constant flow conditions, exogenous adenosine caused dose-related reductions in coronary perfusion pressure described by a maximum reduction in pressure of 30.7 +/- 3.9 mmHg and an ED50 of 977 +/- 813 pmol. Addition of glibenclamide caused a significant (P < 0.01) increase in coronary perfusion pressure of 44.7 +/- 7.2 mmHg and a significant (P < 0.05) rightward shift of the dose-response curve for the depressor effects of adenosine (ED50 = 13.5 +/- 3.8 nmol), with a depression (P < 0.05) of the maximum (16.3 +/- 2.4 mmHg). 5. In conclusion, both KATP and endogenous adenosine make major contributions towards coronary vascular tone and the regulation of coronary flow in the rat isolated heart. Furthermore, in the coronary vasculature a significant proportion of the vasodilator action of adenosine is mediated through the activation of KATP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aversano T., Ouyang P., Silverman H. Blockade of the ATP-sensitive potassium channel modulates reactive hyperemia in the canine coronary circulation. Circ Res. 1991 Sep;69(3):618–622. doi: 10.1161/01.res.69.3.618. [DOI] [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Belloni F. L., Hintze T. H. Glibenclamide attenuates adenosine-induced bradycardia and coronary vasodilatation. Am J Physiol. 1991 Sep;261(3 Pt 2):H720–H727. doi: 10.1152/ajpheart.1991.261.3.H720. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Billman G. E., Avendano C. E., Halliwill J. R., Burroughs J. M. The effects of the ATP-dependent potassium channel antagonist, glyburide, on coronary blood flow and susceptibility to ventricular fibrillation in unanesthetized dogs. J Cardiovasc Pharmacol. 1993 Feb;21(2):197–204. doi: 10.1097/00005344-199302000-00003. [DOI] [PubMed] [Google Scholar]
- Bouchard J. F., Dumont E., Lamontagne D. Evidence that prostaglandins I2, E2, and D2 may activate ATP sensitive potassium channels in the isolated rat heart. Cardiovasc Res. 1994 Jun;28(6):901–905. doi: 10.1093/cvr/28.6.901. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992 Mar;262(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- Dart C., Standen N. B. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993 Nov;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y., Tomoike H., Narishige T., Takahashi T., Kasuya H., Takeshita A. Glibenclamide decreases basal coronary blood flow in anesthetized dogs. Am J Physiol. 1992 Aug;263(2 Pt 2):H399–H404. doi: 10.1152/ajpheart.1992.263.2.H399. [DOI] [PubMed] [Google Scholar]
- Jackson W. F. Arteriolar tone is determined by activity of ATP-sensitive potassium channels. Am J Physiol. 1993 Nov;265(5 Pt 2):H1797–H1803. doi: 10.1152/ajpheart.1993.265.5.H1797. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Codina J., Birnbaumer L., Brown A. M. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990 Sep;259(3 Pt 2):H820–H826. doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- Komaru T., Lamping K. G., Eastham C. L., Dellsperger K. C. Role of ATP-sensitive potassium channels in coronary microvascular autoregulatory responses. Circ Res. 1991 Oct;69(4):1146–1151. doi: 10.1161/01.res.69.4.1146. [DOI] [PubMed] [Google Scholar]
- Lamping K. G., Bloom E. N., Harrison D. G. Regulation of native collateral vessel dilation after coronary occlusion in the dog. Am J Physiol. 1994 Feb;266(2 Pt 2):H769–H778. doi: 10.1152/ajpheart.1994.266.2.H769. [DOI] [PubMed] [Google Scholar]
- Merkel L. A., Lappe R. W., Rivera L. M., Cox B. F., Perrone M. H. Demonstration of vasorelaxant activity with an A1-selective adenosine agonist in porcine coronary artery: involvement of potassium channels. J Pharmacol Exp Ther. 1992 Feb;260(2):437–443. [PubMed] [Google Scholar]
- Miwa A., Jinno Y., Yokoyama T., Izawa T., Ogawa N. Pharmacological analysis of the effect of KRN2391 on coronary vasculature in perfused rat heart. Gen Pharmacol. 1994 May;25(3):471–474. doi: 10.1016/0306-3623(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Moreau R., Komeichi H., Kirstetter P., Yang S., Aupetit-Faisant B., Cailmail S., Lebrec D. Effects of glibenclamide on systemic and splanchnic haemodynamics in conscious rats. Br J Pharmacol. 1994 Jun;112(2):649–653. doi: 10.1111/j.1476-5381.1994.tb13124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae I., Quan L., Sugimoto Y., Tsutamoto T., Kinoshita M. Glibenclamide-induced oscillation of canine coronary artery is independent of myocardial ischemia. J Cardiovasc Pharmacol. 1994 Mar;23(3):473–479. [PubMed] [Google Scholar]
- Narishige T., Egashira K., Akatsuka Y., Katsuda Y., Numaguchi K., Sakata M., Takeshita A. Glibenclamide, a putative ATP-sensitive K+ channel blocker, inhibits coronary autoregulation in anesthetized dogs. Circ Res. 1993 Oct;73(4):771–776. doi: 10.1161/01.res.73.4.771. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991 Dec;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Niiya K., Uchida S., Tsuji T., Olsson R. A. Glibenclamide reduces the coronary vasoactivity of adenosine receptor agonists. J Pharmacol Exp Ther. 1994 Oct;271(1):14–19. [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Randall M. D., McCulloch A. I. The involvement of ATP-sensitive potassium channels in beta-adrenoceptor-mediated vasorelaxation in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1995 Jun;115(4):607–612. doi: 10.1111/j.1476-5381.1995.tb14975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. D., Ujiie H., Griffith T. M. Modulation of vasodilatation to levcromakalim by adenosine analogues in the rabbit ear: an explanation for hypoxic augmentation. Br J Pharmacol. 1994 May;112(1):49–54. doi: 10.1111/j.1476-5381.1994.tb13027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha F. F., Heineman F. W., Ince C., Fleming J., Balaban R. S. ATP-sensitive potassium channel is essential to maintain basal coronary vascular tone in vivo. Am J Physiol. 1992 May;262(5 Pt 1):C1220–C1227. doi: 10.1152/ajpcell.1992.262.5.C1220. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Xu J., Wang L., Hurt C. M., Pelleg A. Endogenous adenosine does not activate ATP-sensitive potassium channels in the hypoxic guinea pig ventricle in vivo. Circulation. 1994 Mar;89(3):1209–1216. doi: 10.1161/01.cir.89.3.1209. [DOI] [PubMed] [Google Scholar]
- von Beckerath N., Cyrys S., Dischner A., Daut J. Hypoxic vasodilatation in isolated, perfused guinea-pig heart: an analysis of the underlying mechanisms. J Physiol. 1991 Oct;442:297–319. doi: 10.1113/jphysiol.1991.sp018794. [DOI] [PMC free article] [PubMed] [Google Scholar]